POINT
Periocular Triamcinolone Versus Intravitreal Triamcinolone Versus Intravitreal Dexamethasone Implant for the Treatment of Uveitic Macular Edema: The PeriOcular versus INTravitreal Corticosteroids for Uveitic Macular Edema (POINT) Trial
|
192
|
National Eye Institute (NEI)/National Institutes of Health (NIH)
(NCT02374060)
|
2018
|
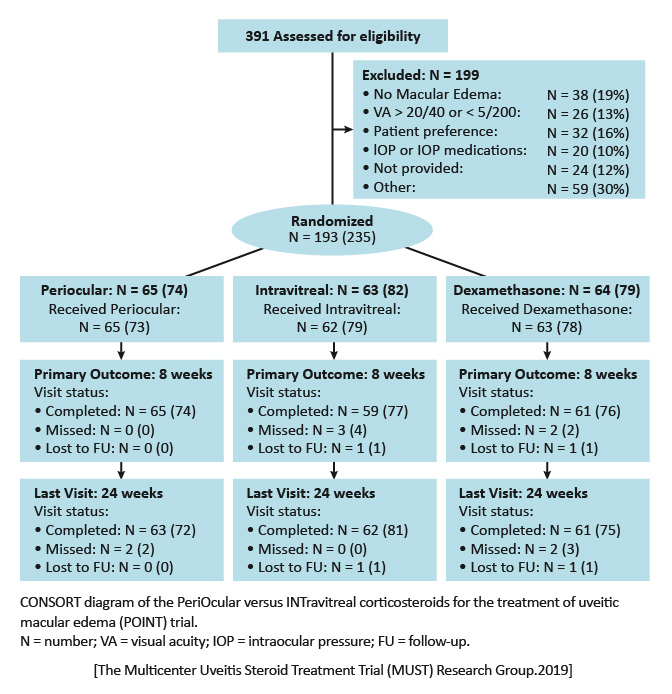
The Multicenter Uveitis Steroid Treatment Trial (MUST) Research Group, Thorne JE, Sugar EA, et al. Periocular triamcinolone versus intravitreal triamcinolone versus intravitreal dexamethasone implant for the treatment of uveitic macular edema: The PeriOcular versus INTravitreal corticosteroids for uveitic macular edema (POINT) Trial. Ophthalmology. 2019;126:283-295. https://www.aaojournal.org/article/S0161-6420(18)31133-3/fulltext
|
|
