| Clinical Study “Common” Name (formal title) | Study design | Treatment arms | Primary outcomes | Results | Author conclusions | "Real-world" impact |
|---|---|---|---|---|---|---|
| ALTAIR [Japanese Treat-and-Extend Study of Aflibercept in Neovascular Age-Related Macular Degeneration (ALTAIR)] | Phase 4, randomized, open label trial |
After receiving 3 monthly doses of aflibercept 2 mg, at Week 16: 2-week adjustment: n=124 (n=108 completed Week 96) 4-week adjustment: n=123 (n=104 completed Week 96) [Ohji.AdvTher.2020] |
Mean change in best-correct visual acuity (BCVA) (Early Treatment of Diabetic Retinopathy Study [ETDRS] letters) from baseline to Week 52 Endpoints were assessed at Week 52 and Week 96. |
Over 52 weeks: 2-week extension group: 7.2 injections; +8.4 letters at Week 52 and +6.1 letters at Week 96 4-week extension group: 6.9 injections; +9.0 letters at Week 52 and +7.6 letters at Week 96 [Ohji.AdvTher.2020] 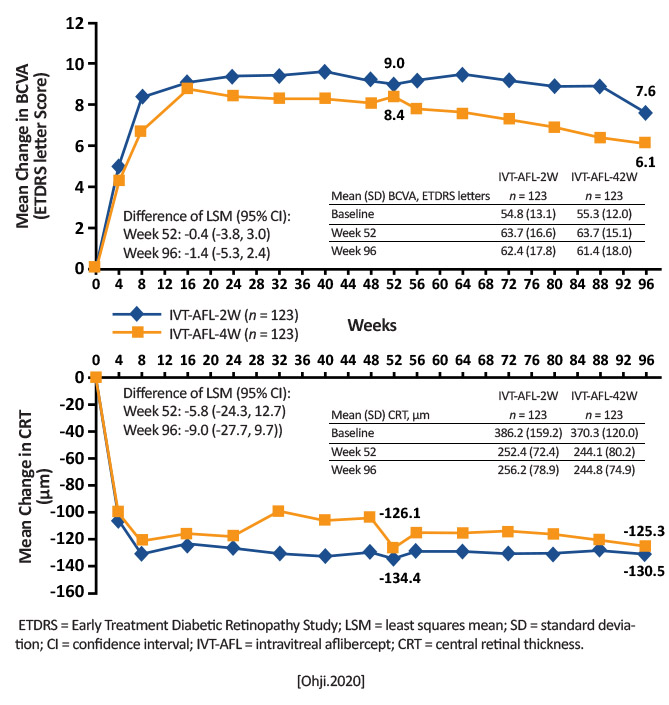
|
Intravitreal aflibercept administered using either 2- or 4-week adjustment treat-and-extend regimens in treatment-nave patients with exudative AMD improved functional (BCVA +9.0 and + 8.4 letters) and anatomic outcomes (central retinal thickness -134.4 and -126.1 m) at 52 weeks; functional and anatomic outcomes were maintained over 96 weeks and were similar between the 2 groups. A larger proportion of patients (35.1% and 40.5%) had an intended injection interval of 16 weeks at Week 52. [Ohji.AdvTher.2020] |
Treatment-nave Japanese patients with wet AMD can be managed safely by extending out the treatment regimen up to every 16 weeks with intravitreal aflibercept. |
| Anchor (Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age-related Macular Degeneration | Multicenter, international, randomized, double-masked, active-treatment-controlled trial. | Verteporfin PDT plus monthly sham intraocular injection (n=143) Sham verteporfin PDT plus monthly intravitreal ranibizumab 0.3 mg (n=140) Sham verteporfin PDT plus monthly intravitreal ranibizumab 0.5 mg (n=140) | Percent of patients losing <15 letters from baseline visual acuity (VA) at month 12. |
<15 letters loss: Ranibizumab 0.3 mg: 94.3%
Ranibizumab 0.5 mg: 96.4%
Verteporfin PDT: 64.3% [Brown.NEJM.2006] |
Both doses of ranibizumab prevented central vision loss and improved mean VA at 1 year. [Brown.NEJM.2016]
Ranibizumab administered monthly is superior in efficacy to PDT with verteporfin in patients with subfoveal, predominantly classic choroidal neovascularization associated with AMD. [Brown.NEJM.2016] |
ANCHOR data led to FDA approval of ranibizumab 0.5 mg for the treatment of wet AMD in 2006, marking the first anti-VEGF approved for this indication. [Lucentis.PI.2018] [Roh.Yanoff.Ophthalmology.2019] |
|
ARCHWAY (A Phase III Study to Evaluate the Port Delivery System With Ranibizumab Compared With Monthly Ranibizumab Injections in Participants With Wet Age-Related Macular Degeneration) |
Phase 3, randomized, open label, visual acuity assessor-masked noninferiority and equivalence trial |
Randomized 3:2 PDS (ranibizumab 100 mg/mL, refill-exchange every 24 weeks): n=251; 248 included in primary analysis Intravitreal ranibizumab (0.5 mg, every 4 weeks): n=167; 167 included in primary analysis |
To determine if the port delivery system (PDS) was noninferior and equivalent to monthly ranibizumab injections in BCVA gains from baseline |
Adjusted mean change from baseline in BCVA score: Averaged over Weeks 36 and 40 PDS: +0.2 ETDRS letters Intravitreal ranibizumab: +0.5 ETDRS letters [Holekamp.Ophthalmology.2021] 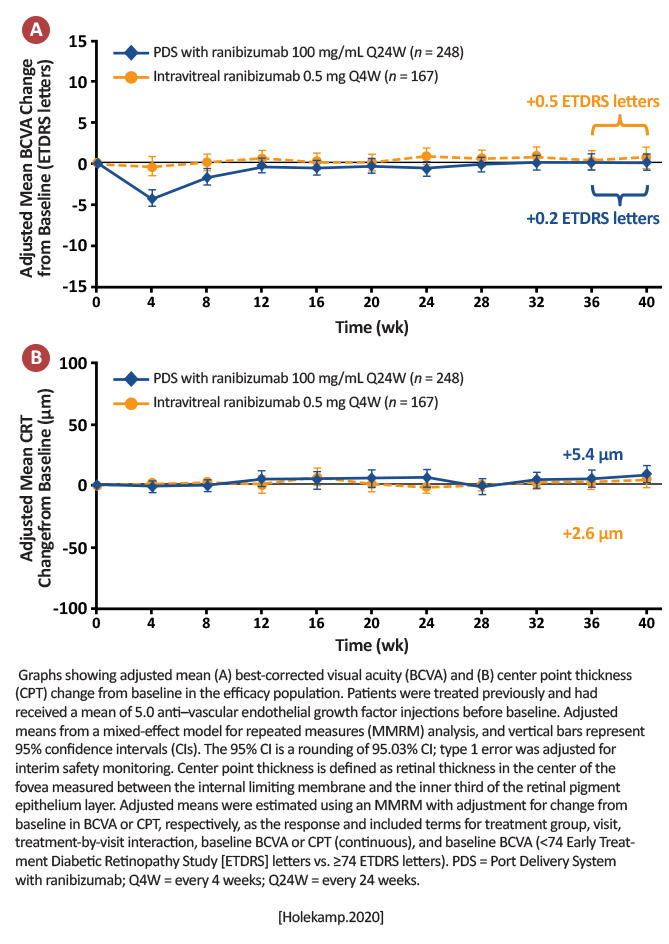
|
Archway met its primary objective and the PDS with fixed refill-exchanges every 24 weeks demonstrated noninferior and equivalent efficacy to monthly ranibizumab, with 98.4% of PDS-treated patients not receiving supplemental treatment in the first 24-week interval. [Holekamp.Ophthalmology.2021] |
Provided the impetus for FDA approval of Susvimo (formerly the port delivery system). The high proportion of patients who did not need additional treatment supports the potential for the PDS to reduce overall treatment and monitoring burden. |
|
AREDS (Age-Related Eye Disease Study) |
Multicenter, randomized, factorial assignment, quadruple masked clinical trial with follow up 7 years | Four treatment arms: Treatment 1: antioxidant formulation (vitamin C 500 mg, vitamin E 400 IU, beta-carotene 15 mg) and zinc (80mg) + copper (2mg) (N=888) Treatment 2: zinc (80mg) + copper (2mg) (N=904) Treatment 3: antioxidant formulation (N=945) Treatment 4: Placebo (N=903) | Change in VA (15-letter decrease) and ocular status (progression to advanced AMD) between treatment groups at 7 year follow up |
Treatment 1: 34% reduced risk of progression when compared to placebo:
Treatment 1: Odds Ratio (OR) 0.72
Treatment 2: OR 0.75
Treatment 3: OR 0.80
[AREDS.2001] |
There is a low risk of developing advanced AMD in patients with intermediate or extensive small-sized drusen. AREDS-type supplements have a moderate benefit in patients at high risk of developing AMD. [Chew.Arch Ophthalmol.2009] |
AREDS-type supplements are recommended for use in patients at high risk of developing advanced AMD due to the potential positive effect on public health. [Chew.Arch Ophthalmol.2009] It was the first large, prospective trial to show a benefit of antioxidant and zinc supplementation to slow the progression of AMD. [Roh.Yanoff Ophthalmology.2019] |
|
AREDS2 (Age-Related Eye Disease Study 2) |
Multicenter, randomized, parallel assignment, double-masked clinical trial |
First randomization:
placebo (N=1,012)
Lutein plus zeaxanthin (10 mg/2mg, N=1,044)
Omega 3s DHA and EPA (650 mg/350 mg; N=1,06) Lutein/zeaxanthin plus DHA/EPA (N=1,079) Second randomization: All patients were offered random assignment of 1 of 4 variations of the original AREDS formulation keeping vitamins C (500 mg), E (400 IU), and copper (2 mg) unchanged while varying zinc and beta-carotene as follows:
|
Progression to advanced AMD determined by centralized grading of annual fundus photographs at mean follow-up of 5 years. | Adding lutein plus zeaxanthin, DHA plus EPA, or both to the AREDS formulation did not further reduce the risk of progressing to advanced AMD. However, more lung cancers were noted in the beta carotene vs no beta carotene group. [AREDS2.JAMA.2013] | Daily supplementation with AREDS2 formulations confirmed a reduction the risk of AMD progression or change VA to the same degree as AREDS1. This allows for the substitution of lutein and zeaxanthin as the carotenoids of choice rather than beta-carotene given the systemic risk of lung cancer in current and former smokers. In addition, omega 3 supplementation did not change outcomes. [AREDS2.JAMA.2013] | AREDS2 provided the information that additional omega3 supplementation was unnecessary to prevent the progression to advanced AMD. In addition, the substitution of the carotenoids (lutein and zeaxanthin were not available at the time of initial formulation) was made to protect current and former smokers from the risk of lung cancer. Lastly it should be remembered that these vitamins prevent the progression to neovascular AMD but not geographic atrophy. |
|
ATLAS (Aflibercept Treat and Extend Therapy for Neovascular Age-related Macular Degeneration) |
Multicenter, prospective, open label, noncomparative, interventional study. | Aflibercept (n=40): baseline, week 4, then extended by 2 weeks, up to a max of 16 week intervals | Change in mean and median best-corrected visual acuity (BCVA) from baseline (58.9 letters) at years 1 and 2 |
Year 1: 7.2 letter gain
Year 2: 2.4 letter gain
[Decroos.AJO.2017]
Mean injections:
Year 1: 8.0
Year 2: 6.5
12-week or longer treatment intervals:
Year 1: 35%
Year 2: 38% [Decroos.AJO.2017] |
Treat-and-extend (TAE) with aflibercept showed significant visual and anatomic gains in year 1, but those gains were slightly diminished in year 2. About 75% of patients could be extended to 8-week intervals. [Decroos.AJO.2017] |
ATLAS was one of the first studies to prospectively report the efficacy of a TAE-style dosing regimen with aflibercept for treatment-nave wet AMD patients. [Decroos.AJO.2017] TAE with aflibercept can be an acceptable treatment regimen for patients with wet AMD without compromising vision. [Decroos.AJO.2017] |
|
AURA (A retrospective non-interventional study to assess the effectiveness of existing Anti-vascular endothelial growth factor [anti-VEGF] treatment Regimens in patients with wet Age-related macular degeneration) |
Observational, retrospective chart review. | Ranibizumab 0.5 mg (n=2,227) | Changes in VA after start of anti-VEGF therapy with ranibizumab 0.5 mg, assessed by ETDRS or Snellen from baseline to 24 months |
Year 1: +2.4 letters
Year 2: +0.6 letters
VA improved to about day 120, then gains were not maintained. [Holz.Br J Ophth.2015] Number of injections: Year 1: 5.0 Year 2: 2.2 [Holz.Br J Ophth.2015] |
Year 1: +2.4 letters
Year 2: +0.6 letters
VA improved to about day 120, then gains were not maintained. [Holz.Br J Ophth.2015] Number of injections: Year 1: 5.0 Year 2: 2.2 [Holz.Br J Ophth.2015] |
Treatment burden is a significant issue for patients with wet AMD and clinicians try to reduce this with dosing regimens that are alternative to the monthly recommendation. This study added to the literature that, although monthly treatments are inconvenient, they could be necessary if the patient was to maximize their vision. Additional research into other treatment schedules and alternative therapies are needed to help reduce the trade-off between frequent treatment and visual outcomes. [Holz.Br J Ophthalmol.2015] |
|
CANTREAT (Canadian Treat-and-Extend Analysis Trial with Ranibizumab in Patients with Neovascular Age-Related Macular Degeneration) |
A randomized, open-label, multicenter, noninferiority intention-to-treat trial |
T&E Ranibizumab 0.5 mg (n=287) Monthly ranibizumab 0.5 mg (n=293) |
Mean change in BCVA in ETDRS letters from baseline to month 24. |
>15 letter gainers:
T&E group: 25.5%
Monthly group 23.1%
>15 letter losers:
T&E group: 6.5%
Monthly group: 5.8% [Kertes.JAMA Opth.2020] |
T&E regimen resulted in clinically meaningful improvement in BCVA that was not worse than monthly treatment with fewer injections and visits. [Kertes.JAMA Opth.2020] |
CANTREAT confirmed the feasibly and effectiveness of the T&E regimen in daily practice. [Kertes.JAMA Opth.2020] In light of the treatment burden, this may help clinicians reduce monthly treatment to a more formal T&E regimen. |
|
CATT (The Comparison of Age-Related Macular Degeneration Treatment Trials) 5-year outcomes |
Multicenter, single-blind, noninferiority trial |
Ranibizumab 0.5 mg every 4 weeks for 1 year; at 1 year, re-randomization to ranibizumab 0.5 mg every 4 weeks or as needed (N= 301)
Bevacizumab 1.25 mg every 4 weeks for 1 year; at 1 year, re-randomization to bevacizumab every 4 weeks or as needed (N = 286) Ranibizumab 0.5 mg as needed for 2 years. (N = 298) Bevacizumab 1.25 mg as needed for 2 years (N = 300) Final follow-up was at 5 years. [CATT.NEJM.2011] |
Change from baseline in VA score at year 2; VA and anatomic outcomes at 5-years | At 5 years: 20/40 or better: 50% 20/200 or worse: 20% [Maguire.Ophthalmology.2016] Mean foveal thickness: 278 m (Change of -20 m from 2 years, and -182 m from baseline) [Maguire.Ophthalmology.2016] | Vision gains with anti-VEGFs are not maintained at 5 years. However, anti-VEGFs are able to preserve vision for a large proportion of patients over the long term. [Maguire.Ophthalmology.2016] |
CATT 5-year follow-up provided the most complete follow-up reported to date on the long-term outcomes for the treatment of neovascular AMD with anti-VEGF drugs. [Maguire.Ophthalmology.2016] But it also found obstacles with long-term use of the current anti-VEGFs, illustrating the need for better agents that can prevent or minimize geographic atrophy. [Maguire.Ophthalmology.2016] |
|
EXCITE (Efficacy and Safety of Ranibizumab in Patients With Subfoveal Choroidal Neovascularization [CNV] Secondary to Age-related Macular Degeneration) |
A 12-month, multicenter, randomized, double-masked, active-controlled study. | Ranibizumab 0.3 mg, dosed quarterly (N=120) Ranibizumab 0.5 mg, dosed quarterly (N=118) Ranibizumab 0.3 mg, dosed monthly ranibizumab. All patients received 3 loading dosing, followed by a 9-month maintenance phase of either monthly or quarterly injections (N=115) | Mean change in BCVA and CRT from baseline to month 12 and the incidence of AEs. |
In per-protocol population (293 pts), BCVA increased from baseline to month 12 by 4.9, 3.8, and 8.3 letters in the 0.3 mg quarterly, 0.5 mg quarterly, and 0.3 mg monthly dosing groups, respectively. [Schmidt-Erfurth.Ophthal.2011] Similar results were observed in the ITT population (353 patients). [Schmidt-Erfurth.Opthal.2011] Mean decrease in CRT from baseline to month 12 in the ITT population was -96.0 ¼m in 0.3 mg quarterly, -105.6 ¼m in 0.5 mg quarterly, and -105.3 ¼m in 0.3 mg monthly group. [Schmidt-Erfurth.Opthal.2011] Most frequent ocular AEs were conjunctival hemorrhage (17.6%, pooled quarterly groups; 10.4%, monthly group) and eye pain (15.1%, pooled quarterly groups; 20.9%, monthly group). There were 9 ocular serious AEs and 3 deaths; 1 death was suspected to be study related (cerebral hemorrhage; 0.5 mg quarterly group). [Schmidt-Erfurth.Opthal.2011] |
After 3 initial monthly ranibizumab injections, both monthly (0.3 mg) and quarterly (0.3 mg/0.5 mg) ranibizumab treatments maintained BCVA in patients with CNV secondary to AMD. At month 12, BCVA gain in the monthly regimen was higher than that of the quarterly regimens. The noninferiority of a quarterly regimen was not achieved with reference to 5.0 letters. The safety profile was similar to that reported in prior ranibizumab studies. The direct comparative analysis between monthly and quarterly treatment regimens of the EXCITE study is consistent with the clinical guidance on ranibizumab treatment, which recommends rigorous monthly monitoring with timely retreatment of patients with recurrent disease activity to achieve the best treatment outcomes for patients. [Schmidt-Erfurth.Ophthal.2011] |
The EXCITE trial was the first study directly comparing visual outcomes between monthly and quarterly dosing regimens in the treatment of patients with subfoveal CNV, secondary to AMD. [Schmidt-Erfurth.Ophthal.2011] EXCITE added to the literature on the optimal dosing regimen for ranibizumab, illustrating that for maximum effectiveness, monthly dosing is preferable. |
|
FIDO (Long-term outcomes in eyes receiving Fixed-Interval Dosing of anti-vascular endothelial growth factor agents for wet age-related macular degeneration) |
Single-practice retrospective chart review. | No treatment; Snellen VA was recorded at baseline and all subsequent injections (N=109). Changes from baseline were calculated at yearly intervals for at least 5 years. | Mean change in letter score at 5 years (n=109) 6 years (n=75) 7 years (n=44) [Peden.Ophth.2014] | Year 5: +14.0 Year 6: +12.2 Year 7: +12.1 [Peden.Ophth.2014] | Continuous fixed-interval dosing results in favorable long-term preservation out to 7 years, with vision stabilizing or improving in 93.2% of eyes. Continuous therapy provides better outcomes than sporadic, as-needed treatment. [Peden.Ophth.2014] | FIDO was the largest database of reported results for patients treated with continuous fixed-interval dosing for more than 5 years. It demonstrated that aggressive treatment with such a dosing regimen can result in superior long-term vision outcomes. FIDO adds to the literature supporting that patients achieve better visual outcomes with continuous, monthly treatment, over as-needed therapy. [Peden.Ophth.2014] |
|
FLUID (A Phase IV, Randomised, Single Masked Study Investigating the Efficacy and Safety of Ranibizumab "Inject and Extend" Using an Intensive Retinal Fluid Retreatment Regimen Compared to a Relaxed Retinal Fluid Retreatment Regimen in Patients With Wet Age-related Macular Degeneration) |
Multicenter, randomized, 24-month, phase 4, single-masked, noninferiority clinical trial. | Intensive group: Ranibizumab 0.5 mg monthly until resolution of subretinal fluid (SRF) and IRF (intraretinal fluid) before extending treatment interval (n=174) Relaxed treatment: Ranibizumab 0.5 mg monthly until resolution of all IRF but tolerant of SRF up to 200 mm at the foveal center before extending the treatment interval (n=175) | Mean change in BCVA and central subfield thickness and number of injections to month 24. |
Intensive group:
|
Ranibizumab 0.5 mg TAE was noninferior to monthly ranibizumab 0.5 mg in resolution of SRF. No new safety concerns were raised. [Guymer.Opthal.2019] |
FLUID continues the debate on the importance of drying patients completely. It is possible that a small amount of SRF can be tolerated, thereby reducing the injection burden in some patients. Residual SRF may also protect patients against atrophy. [Guymer.Opthal.2019] |
| FRB! was the largest series of long-term outcomes of patients receiving VEGF inhibitors for neovascular AMD at the time of publication. [Gilles.Ophth.2017] One of the largest real-world analyses, this showed eyes with worse baseline vision are able to gain, but never catch up to those with better baseline vision. [Gilles.Ophth.2017] More research is necessary to determine if initiating treatment earlier (ie, with better baseline vision) results in greater ability to maintain good vision. |
FRB! (Fight Retinal Blindness!) |
Database observational study | No treatment arms. Outcomes registry (Fight Retinal Blindness) included 1,212 treatment-nave eyes with neovascular AMD that received at least 1 anti-VEGF injection followed over 7 years | Change in mean VA and number of injections and visits. |
Mean VA improved from 55.1 to 61.4 letters after 6 months and remained above the mean presenting VA for about 6 years. [Gilles.Ophth.2017]
After 7 years, mean VA was 2.6 letters lower than baseline for the 131 eyes still being followed. [Gilles.Ophth.2017]
Of those with 20/40 VA before treatment, 40% had lost it after 7 years. [Gilles.Ophth.2017]
Injections/visits:
First Year: 6/9
Years 2-7: 5/7-9 [Gilles.Ophth.2017] |
Although the outcomes were fairly good, 40% of eyes that had VA 20/40 when starting treatment had decreased below this level after 7 years. [Gilles.Ophth.2017] However, the authors attributed geographic atrophy affecting the fovea as the cause of a 10-letter loss after 6.5 years (occurring in 37% of patients). [Gilles.Ophth.2017] |
|
HARBOR (A Phase III, Double-masked, Multicenter, Randomized, Active Treatment-controlled Study of the Efficacy and Safety of 0.5 mg and 2.0 mg Ranibizumab Administered Monthly or on an As-needed Basis [PRN] in Patients With Subfoveal Neovascular Age-related Macular Degeneration) |
Twenty-four-month, multicenter, randomized, double-masked, active treatment-controlled phase 3 trial | Ranibizumab 0.5 mg monthly for 24 months (n=275) Ranibizumab 2.0 mg monthly for 24 months (n= 274) Ranibizumab 0.5 mg as-needed (PRN); 3 loading doses and then ranibizumab 0.5 mg if retreatment criteria were met (n=275) Ranibizumab 2.0 mg PRN; 3 loading doses treated with ranibizumab 0.2 mg if retreatment criteria were met (n=273) |
Primary endpoint: Mean change in BCVA from baseline at month 12. Key secondary endpoint: Mean change in BCVA through month 24 |
Month 12: Ranibizumab 0.5 mg monthly: +10.1 letters Ranibizumab 0.5 mg PRN: +8.2 letters Ranibizumab 2.0 mg monthly: +9.2 letters Ranibizumab 2.0 mg PRN: +8.6 letters [Busbee.Ophthal.2013] At month 24: Ranibizumab 0.5 mg monthly: +9.1 letters Ranibizumab 0.5 mg PRN: +7.9 letters Ranibizumab 2.0 mg monthly: +8.0 letters Ranibizumab 2.0 mg PRN: +7.6 letters [Ho.Ophthal.2014] | Ranibizumab 0.5 mg dosed monthly provides optimum results in patients with wet AMD. Clinically meaningful improvements in VA and anatomic outcomes were seen across all 4 treatment groups. [Busbee.Ophthal.2013] | HARBOR confirmed that ranibizumab 0.5 mg monthly provides similar outcomes to PRN dosing and is not clinically different when strict retreatment criteria are used and may be helpful if patients are unable to maintain a monthly visit schedule. [Busbee.Ophthal.2013] [Ho.Ophthal.2014] A personalized treatment approach is necessary for patients with wet AMD to balance optimal outcomes with a realistic injection schedule. |
|
HAWK (A Two-Year, Randomized, Double-Masked, Multicenter, Three-Arm Study Comparing the Efficacy and Safety of RTH258 Versus Aflibercept in Subjects With Neovascular Age-Related Macular Degeneration) |
Double-masked, multicenter, active-controlled, randomized trials | HAWK/HARRIER: Brolucizumab 6 mg Day 0, Week 4, and Week 8, followed by 1 injection every 8 weeks/1 injection every 12 weeks (q8w/q12w) maintenance regimen until study exit (n=730). Aflibercept 2 mg, single injection at Day 0, Week 4, and Week 8, followed by q8w maintenance regimen until study exit (n=729). (HAWK only): Brolucizumab 3 mg, single injection at Day 0, Week 4, and Week 8, followed by 1 injection every 8 weeks/1 injection every 12 weeks (q8w/q12w) maintenance regimen until study exit (n=358). | Noninferiority in mean BCVA change from baseline to Week 48. | At Week 48 HAWK: Brolucizumab 6 mg: +6.6 letters Brolucizumab 3 mg: +6.1 letters Aflibercept 2.0 mg: +6.8 letters HARRIER: Brolucizumab 6 mg: +6.9 letters Aflibercept 2.0 mg: +7.6 letters [Dugel.Ophthal.2020] | VA gains with brolucizumab dosed with a q12w/q8w regimen were noninferior to aflibercept dosed q8w, while >50% of brolucizumab 6 mg treated eyes were estimated to maintain on q12w dosing immediately after the loading phase through Week 48. [Dugel.Ophthal.2020] | HAWK/HARRIER lead to the FDA approval of brolucizumab (Beovu) for neovascular wet AMD, providing patients with another treatment option. [Beovu.PI.2020] Since the approval of brolucizumab, intraocular inflammation issues have come to light, namely retinal vasculitis and retinal occlusion. In HAWK/HARRIER, 32/730 patients (4%) reported intraocular inflammation, and 6/730 patients (1%) reported retinal artery occlusion. [Beovu.PI.2020] From the time of approval (Oct. 2019) through late Feb. 2020, there were 14 cases of vasculitis (11 of which were occlusive retinal vasculitis) reported from the 46,000 injections. [ASRS.2020] These adverse events led Novartis and the FDA to install a black box warning on the brolucizumab label. [Beovu.PI.2020] |
|
HAWK and HARRIER (HAWK and HARRIER: Ninety-Six-Week Outcomes from the Phase 3 Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration) |
Prospective, randomized, double-masked, multicenter studies | Treatment-nave eyes with nAMD were randomized 1:1:1 to brolucizumab 3 mg (n=358), brolucizumab 6 mg (n=360), aflibercept 2 mg (n=360; HAWK) or 1:1 to brolucizumab 6 mg (n=370), aflibercept 2 mg (n=369; HARRIER). | Mean best-corrected visual acuity (BCVA) change from baseline, proportion of patients on an q12w regimen, retinal thickness, retinal fluid changes, and safety, all to 96 weeks. |
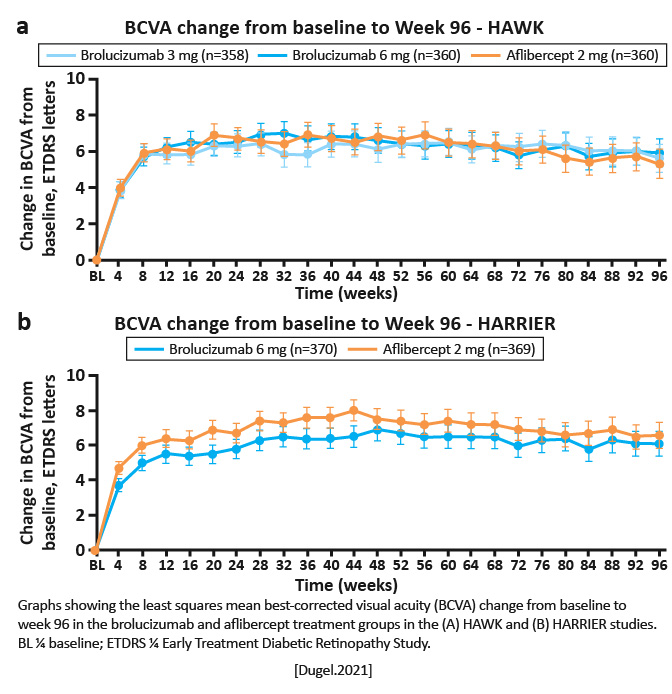
Mean change (least squares [LS] mean standard error) in BCVA from baseline to 96 weeks in HAWK was 5.6 0.79 Early Treatment Diabetic Retinopathy Study (ETDRS) letters for brolucizumab 3 mg, 5.90 0.78 letters for brolucizumab 6 mg, and 5.3 0.78 letters for aflibercept and in HARRIER was 6.1 0.73 letters for brolucizumab 6 mg and 6.6 0.73 letters for aflibercept.
Greater central subfield thickness reductions were observed with brolucizumab 6 mg versus aflibercept in HAWK (LS mean,174.8 ¼m vs 148.7 ¼m; 95% confidence interval for treatment difference, -46.2 to -5.9 ¼m; P= .0115) and HARRIER (LS mean, -197.7 ¼m vs -155.1 ¼m; 95% confidence interval for treatment difference, -62.0 to -23.3 ¼m; P< .0001).
|
Visual outcomes from 48 weeks to 96 weeks confirm the efficacy achieved at 48 weeks. Brolucizumab demonstrated greater fluid resolution compared with aflibercept. The q12w potential for brolucizumab observed at 48 weeks was maintained to 96 weeks. [Dugel.Ophthalmology.2020] | Brolucizumab provides vision gains comparable with those of aflibercept, with greater fluid resolution and a high probability of remaining on an q12w regimen from weeks 48 through 96. These 2-year findings from HAWK and HARRIER demonstrate that brolucizumab may allow for better disease control and reduced treatment burden in nAMD, with an overall well-tolerated safety profile. [Dugel.Ophthalmology.2020] |
|
LUCAS (LUcentis Compared to Avastin Study) |
Multicenter, randomized, noninferiority trial (noninferiority limit of 5 letters). | Ranibizumab 0.5 mg (n=220) Bevacizumab 1.25 mg (n=221) | Change in VA at 1 year | Ranibizumab 0.5 mg: +8.2 letters Bevacizumab 1.25 mg: +7.9 letters [Berg.Ophth.2015] | There was no statistical difference between bevacizumab and ranibizumab at 1 year when using a TAE protocol, but bevacizumab did require more injections. [Berg.Ophth.2015] | The improvement in VA with the TAE regimen was comparable to monthly treatment in CATT. [Berg.Ophth.2015] There was, however, a slightly reduced need for injections in the ranibizumab group (8) compared to bevacizumab (8.9) but not statistically significant. These findings support using Avastin in real-world settings. [Berg.Ophth.2015] |
|
LUMINOUS* (Observe the Effectiveness and Safety of Ranibizumab in Real Life Setting (LUMINOUS) *NOTE: LUMINOUS has multiple publications with different subgroup analyses |
Multicenter, observational study (488 clinical sites in 42 countries [the United States was not included]) |
22,717 patients had nAMD; 16,167 (71.2%) of those were previously treated with ranibizumab Only 1 eye per patient was enrolled |
Mean change in visual acuity (VA) at Year 1, treatment exposure, overall incidence of ocular, nonocular adverse events (AEs) and serious AEs (SAEs) in prior ranibizumab-treated neovascular AMD (nAMD) patients |
Year 1 (n=12,629): Mean VA change from baseline: -1.6 letters Mean number of injections: 4.7 Mean VA change in patients who received 6 injections: -1.8 letters Mean VA change in patients who received >6 injections: +0.5 letters [Holz.PLoSOne.2020] |
Overall, vision was maintained in Year 1 for those previously treated with ranibizumab. Results may help guide clinician recommendations for an appropriate number of injections to achieve optimal visual outcomes. [Holz.PLoSOne.2020] |
LUMINOUS is the largest, prospective, observational, multicenter study on real-world intravitreal ranibizumab use. [Holz.PLoSOne.2020] This study also underscores that treatment effect in real-world settings does not correspond with what was found in the pivotal clinical studies. |
|
MARINA (Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular Age-Related Macular Degeneration) |
Multicenter, 2-year, double-masked, sham-controlled study |
24 monthly intravitreal injections of ranibizumab 0.3 mg (N=238)
24 monthly intravitreal injections of ranibizumab 0.5 mg (N=240)
24 monthly sham injections (N=238) |
Proportion of patients losing < 15 letters from baseline VA at 12 months | At 12 months: Ranibizumab 0.3 mg: 94.5% Ranibizumab 0.5 mg: 94.6% Sham: 62.2% [Rosenfeld.NEJM.2006] At 24 months: Ranibizumab 0.3 mg: 92% Ranibizumab 0.5 mg: 90% Sham: 52.9% [Rosenfeld.NEJM.2006] | Ranibizumab showed both clinically and statistically significant benefits over sham in patients with minimally classic or occult lesions with no classic CNV. [Rosenfeld.NEJM.2006] |
MARINA was the second pivotal trial of ranibizumab (the other being ANCHOR) that led to the approval of ranibizumab for wet AMD. [Lucentis.PI.2018] [Roh.Yanoff.Ophthalmology.2019] Due to the slightly higher rates of arterial thromboembolic events (ATEs) in the higher dose of ranibizumab 0.5 mg versus Ranibizumab 0.3 mg versus sham patients, future clinical trials of anti-VEGF agents now all evaluate ATEs for potential risk to patients. |
|
PIER (A Phase IIIb, Multicenter, Randomized, Double Masked, Sham Injection-Controlled Study of the Efficacy and Safety of Ranibizumab in Subjects With Subfoveal Choroidal Neovascularization [CNV] With or Without Classic CNV Secondary to Age Related Macular Degeneration) |
Phase 3b, multicenter, randomized, double-masked, sham injection-controlled trial | Ranibizumab 0.3 mg (n=60) ranibizumab 0.5 mg (n=61) Sham (n=63) Patients were dosed monthly until the macula was dry, then extended by 2 weeks. If the patient regressed, treatment resumed at a monthly interval until the macula dried again | Mean change from baseline VA at month 12 |
Ranibizumab 0.3 mg: -1.6 letters
Ranibizumab 0.5 mg: -0.2 letters
Sham: -16.3 letters [Abraham.AJO.2010]
At 2 years: Ranibizumab 0.3 mg: -2.2 letters Ranibizumab 0.5 mg: -2.3 letters Sham: -21.4 letters [Abraham.AJO.2010] |
Ranibizumab administered monthly for 3 months and then quarterly provided significant VA benefit to patients with AMD-related subfoveal CNV and was well tolerated. Patients lost 2.3 letters at 1 year from baseline. Ranibizumab did not provide a VA benefit after 14 months without treatment. [Abraham.AJO.2010] | PIER added to the literature confirming that monthly injections are necessary to achieve optimal outcomes and illustrated that patients with wet AMD are at risk of undertreatment. Although there is a great need for alternative dosing strategies in wet AMD, clinicians and patients must continue to strike a balance between treatment burden and visual outcomes. [Abraham.AJO.2010] |
|
PrONTO (Prospective Optical Coherence Tomography (OCT) Study With Lucentis for Neovascular AMD) |
A 2-year prospective, uncontrolled, variable-dosing regimen with intravitreal ranibizumab based on OCT. | Ranibizumab 0.5 mg (N=40). During the first year, retreatment was performed at each monthly visit if any criteria were fulfilled such as an increase in OCT-CRT of at least 100m or a loss of 5 letters or more. During the second year, the retreatment criteria were amended to include retreatment if any qualitative increase in the amount of fluid was detected using OCT. | Mean VA and number of injections at 2 years | At month 24: 11.1 letter gain 212m decrease on OCT-CRT 43% of patients. [Lalwani.AJO.2009] Eyes had an average of 9.9 injections over 24 months. [Lalwani.AJO.2009] |
PrONTO used an OCT-guided variable-dosing regimen with ranibizumab resulting in VA outcomes comparable with monthly dosing while averaging fewer than half the number of injections over 2 years. [Lalwani.AJO.2009] |
Although multiple previous studies illustrated anti-VEGF treatment is most effective dosed monthly, PrONTO was an exception to this. Patients were able to achieve VA outcomes similar to ANCHOR and MARINA with half the number of injections. Importantly, PrONTO only included 40 patients and there was no monthly treatment arm. |
|
SEVEN-UP (Seven-Year Observational Update of Macular Degeneration Patients Post MARINA/ANCHOR and HORIZON Trials) |
Multicenter, noninterventional retrospective cohort study | Cross-sectional cohort study of exudative AMD patients 7 or more years after initiation of the intravitreal ranibizumab regimen in the treatment arms of the pivotal ANCHOR or MARINA studies, who had subsequent follow up in the HORIZON Study. | Percentage with BCVA of 20/70 or better | 7 years post-ANCHOR/MARINA: About half of the eyes were stable About 33% maintained good visual outcomes About 33% had >15 letter decline [Rofagha.Ophthal.2013] | The results of the SEVEN-UP study point to poor long-term outcomes among these AMD patients, with a significant portion at risk for ongoing vision loss many years after treatment initiation. [Rofagha.Ophthal.2013] | SEVEN-UP showed that over time, patients will continue to lose vision, in some cases to worse-than-baseline. Patients who initially fare well may lose vision over the long-term, re-emphasizing the need for frequent visits. Those who received the highest quartile of injections within 7.1 years manifested an improvement in VA over baseline, demonstrating that continuous treatment is necessary for long term success. A key reason for vision loss was macular atrophy and therefore this study questioned whether long-term anti-VEGF use can cause macular atrophy. [Rofagha.Ophthal.2013] |
|
TENAYA/LUCERNE (Efficacy, Durability, and Safety of Intravitreal Faricimab Up to Every 16 Weeks for Neovascular Age-Related Macular Degeneration (TENAYA and LUCERNE): Two Randomised, Double-Masked, Phase 3, Non-Inferiority Trials) |
Randomized, double-masked, non-inferiority trials across 271 sites worldwide | Random 1:1 assignment of intravitreal faricimab 6.0 mg up to every 16 weeks, based on protocol-defined disease activity assessments at weeks 20 and 24, or aflibercept 2.0 mg every 8 weeks. | Mean change in best-corrected visual acuity (BCVA) from baseline averaged over weeks 40, 44, and 48 (prespecified non-inferiority margin of four letters), in the intention-to-treat population with neovascular AMD (nAMD). |
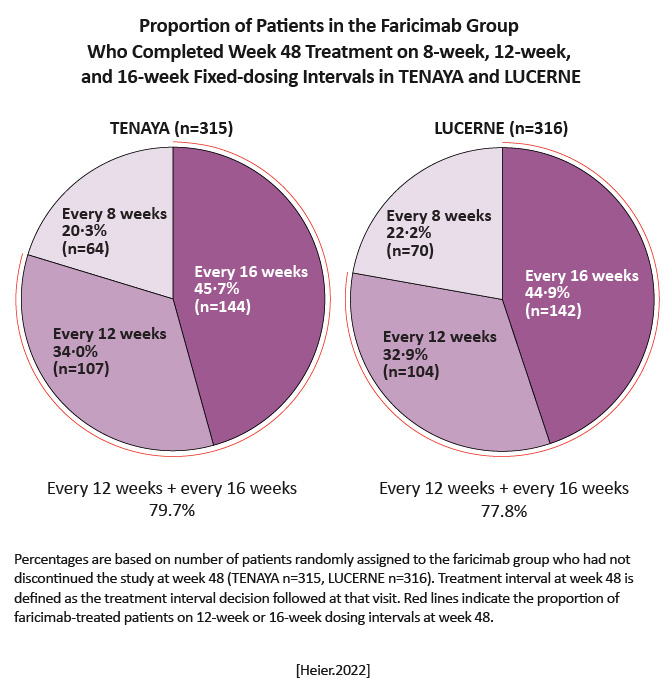
BCVA change from baseline with faricimab was non-inferior to aflibercept in both TENAYA (adjusted mean change 5.8 letters [95% CI, 4.6 to 7.1] and 5.1 letters [3.9 to 6.4]; treatment difference 0.7 letters [1.1 to 2.5]) and LUCERNE (6.6 letters [5.3 to 7.8] and 6.6 letters [5.3 to 7.8]; treatment difference 0.0 letters [-1.7 to 1.8]).
Rates of ocular adverse events were comparable between faricimab and aflibercept (TENAYA n=121 [36.3%] vs n=128 [38.1%], and LUCERNE n=133 [40.2%] vs n=118 [36.2%]).
|
Visual benefits with faricimab given at up to 16-week intervals demonstrate its potential to meaningfully extend the time between treatments with sustained efficacy, thereby reducing treatment burden in patients with nAMD. [Heier.Lancet.2022] | Disease control afforded by the novel dual pathway inhibition with faricimab could allow extending time between treatments while maximizing vision gains, addressing a key clinical unmet need for durable therapies in management of nAMD. [Heier.Lancet.2022] |
|
TREND (Treat-and-Extend versus Monthly Regimen in Neovascular Age-Related Macular Degeneration) |
A 12-month phase 3b VA assessor-masked, multicenter, randomized, interventional study. | Ranibizumab 0.5 mg T&E (n= 323) Ranibizumab 0.5 mg monthly regimen (n=327) | Change in BCVA from baseline to month 12 | Ranibizumab 0.5 mg T&E: +6.2 letters Ranibizumab 0.5 mg monthly: +8.1 letters [Silva.Ophthal.2018] | Ranibizumab 0.5 mg administered according to a T&E dosing regimen was noninferior and clinically comparable with a monthly regimen in improving VA from baseline to the end of study. [Silva.Ophthal.2018] | TREND is another study showing that although monthly injections leads to better visual outcomes, patients can be treated with T&E and achieve good outcomes while reducing the extensive treatment burden. It further illustrates the importance of working with a patient directly to personalize their treatment plan based on their priorities and needs. |
|
T-REX (Treat & Extend Treatment With 0.5mg Ranibizumab vs Monthly Treatment With 0.5mg Ranibizumab) |
Phase 3b, multicenter, randomized, controlled study |
Ranibizumab 0.5 mg monthly (n=20) Ranibizumab 0.5 mg TREX (follow-up intervals are increased when there is no clinical and SD-OCT evidence of disease activity by 2-week intervals and patients are treated at every visit) |
Mean change in BCVA from baseline | At 2 years: Ranibizumab 0.5 mg monthly: +10.5 letters Ranibizumab 0.5 mg TREX: +8.7 letters [Wykoff.Ophth.2017] At year 3: Ranibizumab 0.5 mg monthly: Decline to +5.4 letters over baseline Ranibizumab 0.5 mg TREX, continued on TREX: Decline to +5.0 letters from baseline Ranibizumab 0.5 mg TREX, continued to PRN: -6.7 letters from baseline [Wykoff.BrJ Ophth.2018] | At year 3, monthly to PRN or continual TREX dosing provided better visual outcomes than TREX transitioning to PRN. [Wykoff.BrJ Ophth.2018] | TREX continues the conversation on optimal dosing for wet AMD patients and why some patients may not achieve ideal VA outcomes. The authors hypothesize that the poor outcomes seen in year 3 TREX may not be related to undertreatment, but that eyes that do not manifest ongoing CNV activity could possibly be severely impaired and perhaps prone to develop atrophy. [Wykoff.BrJ Ophth.2018] TREX re-affirms the need for vigilant monitoring (even without dosing) in patients with wet AMD. |
|
VIEW1/VIEW2 (Vascular Endothelial Growth Factor [VEGF] Trap-Eye: Investigation of Efficacy and Safety in Wet Age-Related Macular Degeneration) |
Double-masked, multicenter, parallel-group, active-controlled, randomized trials. | Ranibizumab 0.5 mg (n=595) Aflibercept 2.0 mg every 4 weeks (n=613) Aflibercept 0.5 mg (n=601) Aflibercept 2.0 mg every 8 weeks (including 1 additional 2.0 mg dose at Week 4) for the first year. (n=610) | Noninferiority (margin of 10%) of the aflibercept regimens to ranibizumab in the proportion of patients maintaining vision at week 52 (losing <15 letters on ETDRS chart). |
At week 52, percentage of patients losing <15 letters (VIEW1/VIEW2):
Ranibizumab: 94.4%/94.4%
Aflibercept 2.0 q4: 95.1%/95.6%
Aflibercept 0.5 q4: 95.9%/96.3%
Aflibercept 2.0 q8: 95.1%/95.6% [Heier.Ophthal.2012] |
Aflibercept dosed q4 weeks or q8 weeks after 3 initial monthly doses was noninferior to ranibizumab. [Heier.Ophthal.2012] |
VIEW 1 and VIEW 2 showed aflibercept was statistically noninferior to ranibizumab in its ability to maintain vision when dosed monthly or every 2 months. An interesting subanalysis showed that those with poorer anatomic outcomes at 12 weeks fared better with aflibercept every 4 weeks than every 8 weeks. [Heier.Ophthal.2012] |