| Clinical Study “Common” Name (formal title) | Study design | Treatment arms | Primary outcomes | Results | Author conclusions | “Real-world” impact |
|---|---|---|---|---|---|---|
| ACCORD (Action to Control Cardiovascular Risk in Diabetes) / Effects of Medical Therapies on Retinopathy Progression in Type 2 Diabetes | Randomized trial | Participants (n=10,251) with type 2 diabetes who were at high risk for cardiovascular disease received intensive or standard treatment for glycemia and also for dyslipidemia (160 mg daily of fenofibrate plus simvastatin or placebo plus simvastatin) or for systolic blood-pressure control (target, <120 or <140 mm Hg). A subgroup (n=2856) was evaluated for the effects of these interventions at 4 years on the progression of diabetic retinopathy by 3 or more steps on the Early Treatment Diabetic Retinopathy Study (ETDRS) Severity Scale or the development of diabetic retinopathy that required laser photocoagulation or vitrectomy. | Composite end point of progression of diabetic retinopathy by at least three steps on the ETDRS Severity Scale or development of proliferative diabetic retinopathy that required photocoagulation therapy or vitrectomy. | Rates of diabetic retinopathy progression were 7.3% with intensive glycemia treatment versus 10.4% with standard therapy; 6.5% with fenofibrate for intensive dyslipidemia therapy versus 10.2% with placebo; and 10.4% with intensive blood-pressure therapy versus 8.8% with standard therapy. | Diabetic retinopathy progression was reduced by intensive glycemic control and intensive combination treatment of dyslipidemia but not intensive blood-pressure control. | Intensive glycemia therapy has a positive effect on retinopathy progression in those with newly diagnosed type 2 diabetes or those who did not yet have hypertension, lipid abnormalities, or established cardiovascular disease. Fenofibrate, when added to statin therapy, slows the progression of diabetic retinopathy in patients with type 2 diabetes. [ACCORD Study Group.N Engl J Med.2010] |
|
BOULEVARD [A Multiple-Center, Multiple-Dose, Randomized, Active Comparator-Controlled, Double-Masked, Parallel Group, 36-Week Study to Investigate the Safety, Tolerability, Pharmacokinetics, and Efficacy of RO6867461 Administered Intravitreally in Patients With Diabetic Macular Edema] |
Prospective, randomized, active comparator-controlled, double-masked, multicenter, phase 2 study |
Three arms: Treatment-naïve (n = 168): Faricimab 6 mg (n = 55) Faricimab 1.5 mg. (n = 54) Ranibizumab 0.3 mg (n = 59) Previously treated (n = 61): Faricimab 6 mg (n = 29) Faricimab 1.5 mg (n = 1) Ranibizumab 0.3 mg (n = 31) [Sahni.Ophthal.2019] |
Mean change in BCVA from baseline to week 24 for faricimab compared to ranibizumab in treatment-naïve patients |
Mean improvement from baseline to week 24: Treatment-naïve (n = 168): Faricimab 6 mg: 13.9 letters (80% CI, 12.2-15.6 ETDRS letters) (P = .03 compared to ranibizumab) Faricimab 1.5 mg: 11.7 letters (80% CI, 10.1-13.3 letters) Ranibizumab 0.3 mg: 10.3 letters (80% CI, 8.8- 11.9 ETDRS letters) 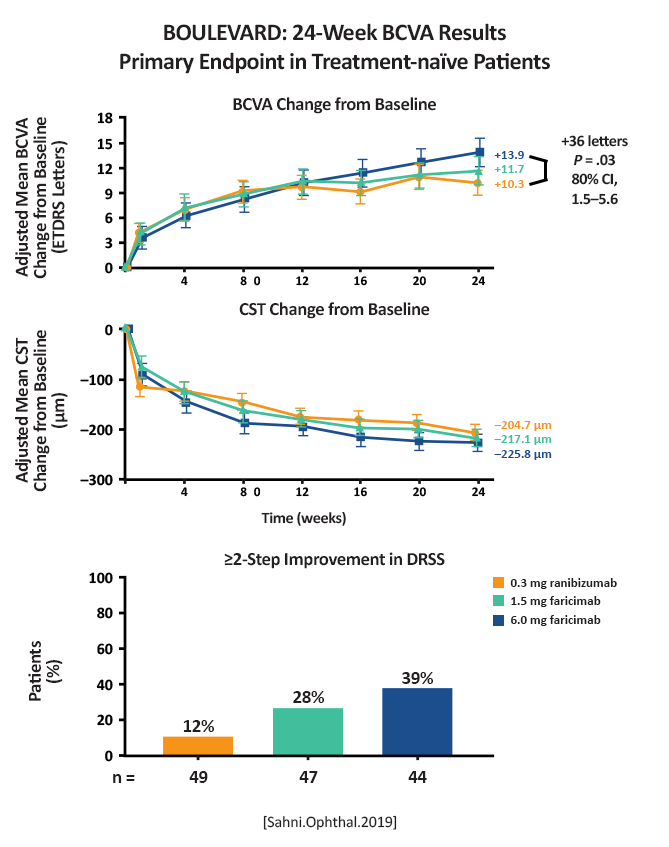 (Figure 3) [Sahni.Ophthal.2019] Previously treated: 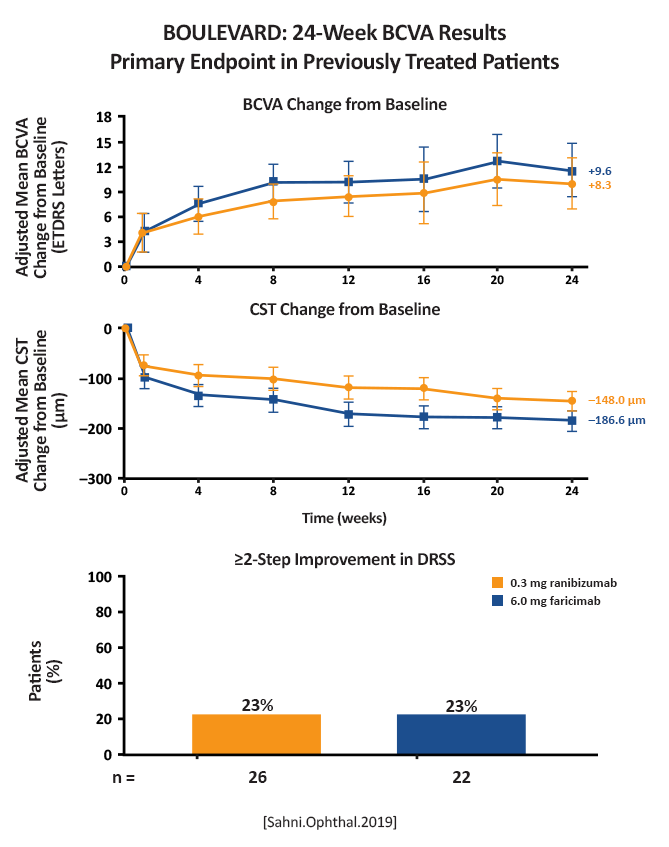 (Figure 5) [Sahni.Ophthal.2019] |
Faricimab, the first bispecific antibody specifically designed for intraocular use, binds and neutralizes both Ang-2 and VEGF-A. In the BOULEVARD phase 2 randomized clinical trial for DME, faricimab met its primary end point, demonstrating clinically meaningful and superior visual acuity gains compared with ranibizumab. [Sahni.Ophthal.2019] |
This is the first study to show a bispecific antibody can be efficacious for the treatment of DME. The positive results from BOULEVARD led to 2 larger phase 3 studies, YOSEMITE and RHINE. |
| CLARITY (Clinical Efficacy of Intravitreal Aflibercept Versus Panretinal Photocoagulation for Best Corrected Visual Acuity in Patients with Proliferative Diabetic Retinopathy at 52 Week: A Multicentre, Single-Blinded, Randomised, Controlled, Phase 2b, Noninferiority Trial) | Multicenter, single-blinded, randomized, controlled, noninferiority clinical trial |
Per-protocol group: Aflibercept, 2 mg/0.05 mL (n=104) Panretinal photocoagulation standard care (n=106) Modified intention-to-treat group: Aflibercept, 2 mg/0.05 mL (n=112) Panretinal photocoagulation standard care (n=109) |
Change in best-corrected visual acuity at 52 weeks with a linear mixed-effect model that estimated adjusted treatment effects at both 12 weeks and 52 weeks. | Aflibercept was non-inferior and superior to panretinal photocoagulation in both the modified intention-to-treat population (mean best- corrected visual acuity difference, 3.9 letters) and the per-protocol population (4.0 letters). | The anti-vascular endothelial growth factor aflibercept showed noninferiority compared with panretinal photocoagulation. Patients preferred aflibercept over panretinal photocoagulation in a clinical setting. | This was the second study to show non-inferiority of anti-VEGF therapy to panretinal photocoagulation and the first study to show potential advantage in best-corrected visual acuity versus panretinal photocoagulation with an anti-VEGF agent, in this case, aflibercept. [Sivaprasad.Lancet.2017] |
| FIELD (The Fenofibrate Intervention and Event Lowering in Diabetes)/ Effect of Fenofibrate on the Need for Laser Treatment for Diabetic Retinopathy: A Randomised Controlled Trial | Randomized, trial |
In the initial FIELD study: Fenofibrate 200 mg/day (n=4895). Placebo (n=4900). A substudy of 1012 patients had retina photographs taken and were graded with ETDRS criteria to determine the cumulative incidence of diabetic retinopathy and its component legions. |
Laser treatment was needed more often in participants with poorer glycemic or blood pressure control than in those with good glycemic or blood pressure control. The need for treatment was not affected by plasma lipid concentrations. | Fenofibrate treatment in individuals with type 2 diabetes reduced the need for laser treatment for diabetic retinopathy. | The use of fenofibrate lowered the need for laser treatment among those with type 2 diabetes. The mechanism of this effect does not seem to be related to plasma concentration of lipids. | Fenofibrate, in addition to other treatments for hyperglycemia and other risk factors for retinopathy reduces the need for laser treatment in individuals with type 2 diabetes. The question remains – is fenofibrate outcome the disease due to its ability to lower lipids or due to its anti-inflammatory effect. [Keech.AC.Lancet.2007] |
| MEAD (A Study of the Safety and Efficacy of a New Treatment for Diabetic Macular Edema) | Randomized, controlled trial |
Dexamethasone (DEX) 0.7 mg implant: n=351 DEX 0.35 mg implant: n=347 Sham: n=350 |
Achievement of ≥15-letter improvement in best-corrected visual acuity (BCVA) from baseline at study end |
Percentage of patients with ≥15 letter gain: DEX 0.7 mg: 22.2% DEX 0.35 mg: 18.4% Sham: 12.0% (P< .001 between DEX 0.7 mg and sham; P≤ .018 between DEX 0.35 and sham) Mean number of treatments over 3 years: DEX 0.7 mg: 4.1 DEX 0.35 mg: 4.4 Sham: 3.3 [Boyer.Ophthalmology.2014] 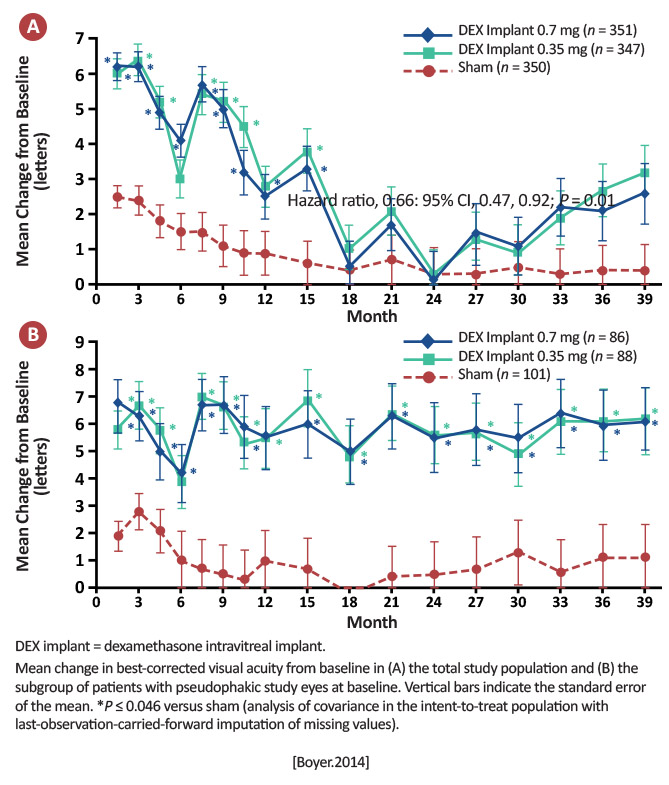
|
With an average of only 4 to 5 injections over 3 years, patients treated with DEX implant achieved statistically significant and clinically meaningful visual improvements. These data support the use of DEX implant in the management of patients with DME. [Boyer.Ophthalmology.2014] |
The use of the DEX implant can result in good vision gains, with a much-reduced treatment schedule than anti-vascular endothelial growth factors (VEGFs), but caution should be urged before treating those who are phakic. |
|
PHOTON [A Randomized, Double-Masked, Active-Controlled Phase 2/3 Study of the Efficacy and Safety of High-Dose Aflibercept in Patients With Diabetic Macular Edema] |
Multi-center, randomized, double-masked study in patients with DME (both treatment-naïve and previously treated) Randomized 1 (2q8) : 2 (8q12) : 1 (8q16)Multi-center, randomized, double-masked study in patients with DME (both treatment-naïve and previously treated) Randomized 1 (2q8) : 2 (8q12) : 1 (8q16) |
Three arms: 2q8 (n = 167): Received 5 initial monthly injections of aflibercept, then aflibercept 2 mg every 8 weeks 8q12 (n = 328): Received 3 initial monthly injections, then aflibercept 8mg every 12 weeks 8q16 (n = 163): Received 3 initial monthly injections, then aflibercept 8mg every 16 weeks |
Primary end point at week 48: Mean change in BCVA (non-inferiority) |
BCVA change at week 48: 2q8: + 9.2 letters 8q12: + 8.8 letters 8q16: + 7.9 letters 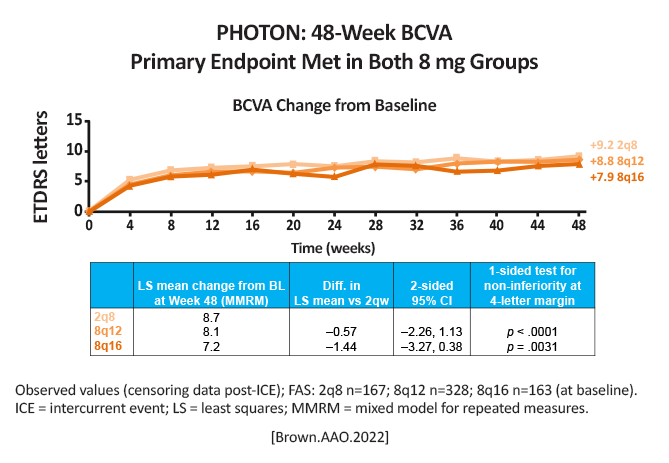 [Brown.AAO.2022] pg 12 Difference in LSmeans compared to 2q8: 8q12: (-0.57 [95% CI, -2.26, 1.13]), P < .0001 8q16: (-1.44 [95% CI, -3.27, 0.39]), P = .0031 93% of 8 mg patients maintained dosing intervals ≥ 12 weeks |
Aflibercept 8mg dosed every 12 or 16 weeks was non-inferior compared to aflibercept 2mg dosed every 8 weeks. Despite fewer initial monthly doses, 8 mg exhibited longer duration at each matched interval, thus achieving similar retinal thickness to 2 mg by Week 48 [Brown.AAO.2022] |
A higher dose of aflibercept (8 mg) produced comparable results as the traditional lower dose (aflibercept 2 mg), without any new safety concerns. This means patients can extend the timing of their visits, and this could also potentially reduce clinic treatment burden. |
|
Protocol AC (Aflibercept Monotherapy or Bevacizumab First for Diabetic Macular Edema) |
Multicenter, randomized trial at 54 clinical sites in the US | 158 eyes were assigned to receive aflibercept monotherapy; 154 to receive bevacizumab first. | Mean change in visual acuity over 2 years among adults with diabetic macular edema involving the macular center and a visual letter score of 24 to 69 on a scale from 1 to 100. |
Over the 2-year period, 70% of the eyes in the bevacizumab-first group were switched to aflibercept therapy. Mean improvement in visual acuity was 15.0 letters in the aflibercept-monotherapy group and 14.0 letters in the bevacizumab first group (P=.37). At 2 years, the mean changes in visual acuity and retinal central subfield thickness were similar in the two groups. 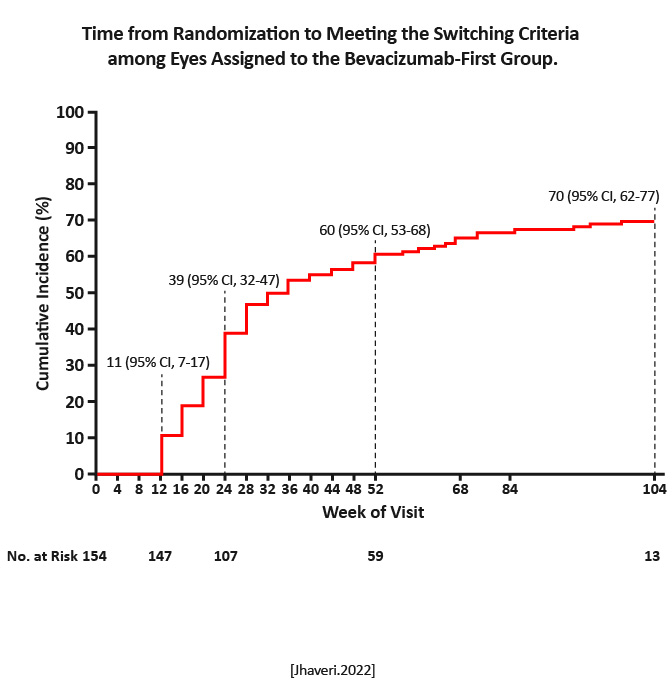
|
There was no evidence of a significant difference in visual outcomes over a 2-year period between aflibercept monotherapy and treatment with bevacizumab first with a switch to aflibercept in the case of suboptimal response. [Jhaveri.NEJM.2022] |
Findings from this trial are particularly relevant given the increasing frequency of insurers mandating step therapy with bevacizumab before the use of other drugs that have been approved by the FDA. However, the results from this trial can be generalized only to patients who receive therapy according to the same switching criteria, anti-VEGF agents, and retreatment algorithm as were used in this trial. Given the difference between the drugs in cost, the initiation of treatment with bevacizumab could have substantial cost reductions for the healthcare system. [Jhaveri.NEJM.2022] |
| Protocol V (Treatment for CI-DME in Eyes With Very Good VA Study [Protocol V]) | Randomized trial |
Intravitreal aflibercept 2.0 mg: n=226 Focal/grid laser photocoagulation: n=240 Observation: n=236 |
Comparing vision loss from baseline to 2 years among eyes managed with intravitreal aflibercept, laser photocoagulation, or observation in patients with center-involved diabetic macular edema (CI-DME) and good vision (20/25 or better) |
Eyes with visual acuity (VA) that decreased 5 letters from baseline: Aflibercept: 16% Photocoagulation: 17% Observation: 19% [Baker.JAMAO.2019] 
|
There was no significant difference among the groups in vision loss at 2 years, suggesting observation without treatment unless VA worsens is a reasonable strategy for CI-DME. [Baker.JAMAO.2019] |
For people with CI-DME and good vision, observation is a viable strategy that is unlikely to result in more letters lost than treatment with either laser or anti-VEGF. |
| The Diabetic Retinopathy Study Research Group. Preliminary Report on Effects of Photocoagulation Therapy. | Randomized, controlled trial |
Immediate photocoagulation (n=1727), followed by random assignment to use of an argon laser (n=858) or a xenon arc photocoagulator (n=869). One eye of each patient was selected for treatment and the other eye for nontreatment. |
Visual acuity of less than 5/200 at one or more, two or more, or three or more consecutively completed 4-month visits | Visual acuity of less than 5/200 at two or more follow-up visits: 9.4% of untreated eyes and 4.1% of treated eyes | Photocoagulation can help prevent progression to severe vision loss in eyes with severe retinopathy. |
Photocoagulation performed per the study protocol (extensive “scatter” photocoagulation and focal treatment of new vessels) can help prevent severe vision loss over a 2-year follow-up period in eyes with severe retinopathy. [DRSRG.1976] |
|
The Protocol W Randomized Clinical Trial (Effect of Intravitreous Anti–Vascular Endothelial Growth Factor vs Sham Treatment: The Protocol for Diabetic Retinopathy: The Protocol W Randomized Clinical Trial) |
Randomized trial |
2.0 mg of aflibercept injections (n = 200) or sham (n = 199) given at baseline; 1, 2, and 4 months; and every 4 months through 2 years. Between 2 and 4 years, treatment was deferred if the eye had mild NPDR or better. Aflibercept was administered in both groups if center-involved-DME with vision loss (≥10 letters at 1 visit or 5-9 letters at 2 consecutive visits) or high-risk (PDR) developed. |
Among those with nonproliferative diabetic retinopathy (NPDR), development of center-involved diabetic macular edema (DME) with vision loss or proliferative diabetic retinopathy through May 2020, when the last 2-year visit was completed. |
The 2-year cumulative probability of developing center-involved -DME with vision loss or PDR was 16.3% with aflibercept vs 43.5% with sham. Overall hazard ratio for either outcome was 0.32 (97.5% CI, 0.21-0.50; P < .001), favoring aflibercept. The 2-year cumulative probability of developing PDR was 13.5% in the aflibercept group vs 33.2% in the sham group; the 2-year cumulative probability of developing CI-DME with vision loss was 4.1% in the aflibercept group vs 14.8% in the sham group. 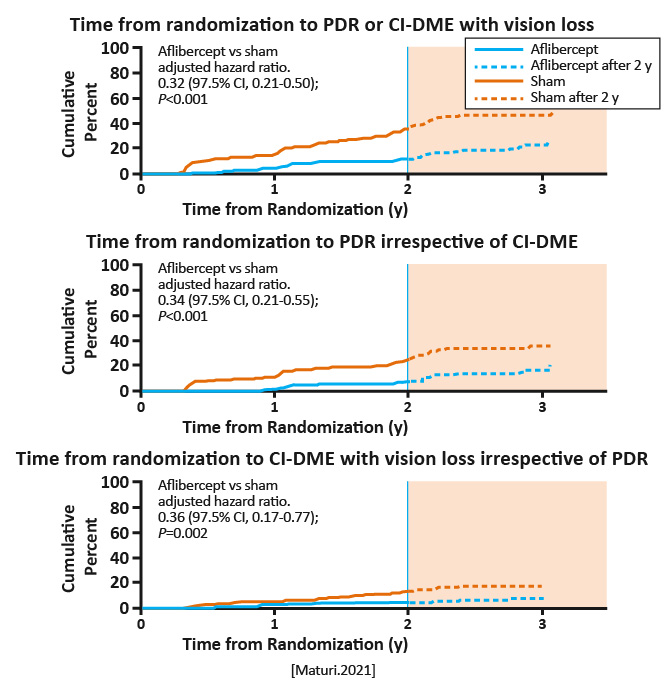
|
Among eyes with moderate to severe NPDR, the proportion of eyes that developed PDR or vision-reducing center-involved -DME was lower with periodic aflibercept compared with sham treatment. However, through 2 years, preventive treatment did not confer visual acuity benefit compared with observation plus treatment with aflibercept only after development of PDR or vision-reducing center-involved DME. [Maturi.JAMA Ophthalmol.2021] |
Through 2 years, preventive treatment with aflibercept did not confer visual benefit, on average, compared with initial observation and intravitreal anti-VEGF therapy given only after PDR or DME development. The 4-year results will be critical to assess whether PDR and DME prevention with aflibercept results in long-term VA benefit. [Maturi.JAMA Ophthalmol.2021] |
| VIVID/VISTA (Study of Intravitreal Aflibercept Injection [IAI; EYLEA®; BAY86-5321] in Patients With Diabetic Macular Edema [VISTA DME]) Intravitreal Aflibercept Injection in Vision Impairment Due to DME (VIVID-DME) | Phase 3, randomized, double-masked, active-controlled trials (2) |
VISTA: Laser: n=154 Intravitreal aflibercept (IAI) every 4 weeks (2q4): n=154 IAI every 8 weeks (2q8): n=151 VIVID: Laser: n=132 IAI 2q4: n=136 IAI 2q8: n=135 In both studies: Aflibercept 2 mg (2q4): injections every 4 weeks from baseline to Week 48, and sham laser at baseline and at visits at which eyes met the criteria for laser retreatment (starting at Week 12 and no more often than every 12 weeks) Aflibercept 2 mg (2q8): injections at baseline, Week 4, Week 8, Week 12, and Week 16, followed by aflibercept 2 mg every 8 weeks from Week 24 to Week 48. Eyes in this group received sham injections, starting at Week 20, on alternating visits when they were not scheduled to receive an active injection. |
Mean BCVA gains from baseline to Week 52 for patients with center-involved diabetic macular edema (CI-DME) |
Mean BCVA gains from baseline to Week 52: VISTA [% of ≥15 letter gains] Aflibercept 2q4: 12.5 letters [41.6%] Aflibercept 2q8: 10.7 letters [31.1%] Laser: 0.2 letters [7.8%] (P< .0001) VIVID Aflibercept 2q4: 10.5 letters [32.4%] Aflibercept 2q8: 10.7 letters [33.3%] Laser: 1.2 letters [9.1%] (P< .0001) [Korobelnik.Ophthalmology.2014] 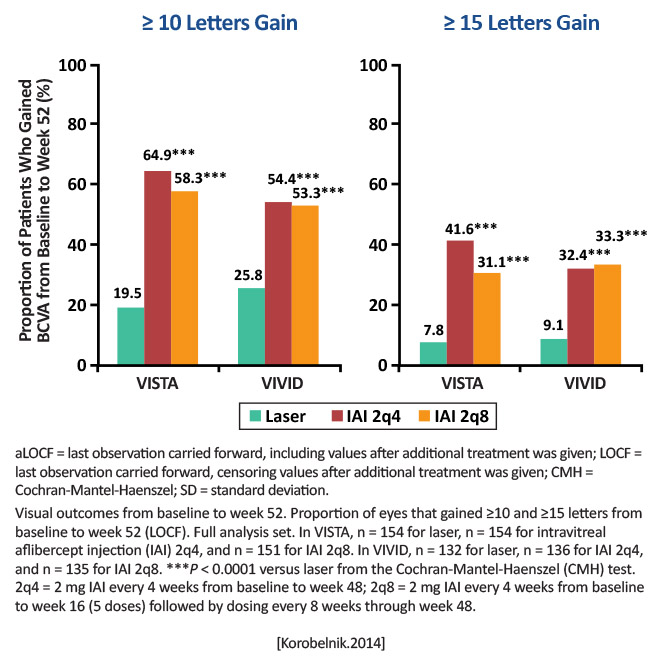
|
The 1-year results of the VISTA/VIVID studies demonstrate that IAI delivered every 4 or every 8 weeks (after 5 initial monthly doses) significantly improved visual outcomes and significantly decreased severe vision loss, while simultaneously improving the diabetic retinopathy severity score, compared with focal laser photocoagulation. [Korobelnik.Ophthalmology.2014] |
First phase 3 studies directly comparing aflibercept to laser in DME. [Korobelnik.Ophthalmology.2014] The authors also noted the trials included a greater percentage of ethnicities than other anti-VEGF DME studies. These findings add to the growing literature that intravitreal anti-VEGF agents are able to provide good anatomic and vision gains in people with DME, and in CI-DME. |