| Clinical study "common" name (formal title) | Primary outcomes | Treatment arms | Results | Author conclusions | "Real-world" impact |
|---|---|---|---|---|---|
| BRILLIANCE (Ranibizumab vs Verteporfin Photodynamic Therapy in Asian Patients With Myopic Choroidal Neovascularization) | Mean best corrected visual acuity (BCVA) change from baseline to Month 12 (primary outcome at Month 3) |
12-month, phase 3, randomized, double-masked, multicenter Group 1 (ranibizumab 0.5 mg; VA stabilization): n=182 Group 2 (ranibizumab 0.5 mg; disease activity): n=184 Group 3 (verteporfin with photodynamic therapy [vPDT] eligible for ranibizumab after Month 3): n=91 |
Change in BCVA from baseline to Month 1 through Month 3: Group 1: +9.5 letters Group 2: +9.8 letters Group 3: +4.5 letters Anatomic changes in central subfield thickness were rapid and clinically relevant in the ranibizumab arms and held through Month 12. Anatomic changes in the vPDT group decreased at Month 1 and stabilized through Month 3; a further decrease was noted only after ranibizumab was allowed. [Chen.Retina.2013] 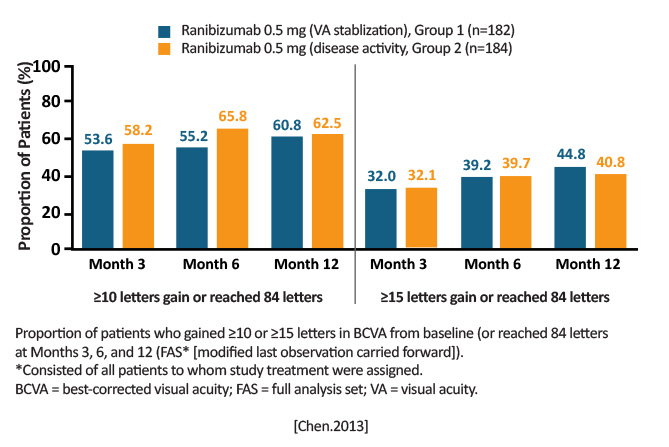
|
Ranibizumab was superior to vPDT in Asian patients (primarily Chinese) at Month 3, and treatment effects lasted through Month 12.
Consistent with findings from the RADIANCE study, ranibizumab retreatment guided by disease activity was noninferior to retreatment guided by visual acuity stabilization criterion. [Chen.Retina.2019] |
Similar results to RADIANCE (Asian and white populations), suggesting ranibizumab efficacy in treating mCNV is not hindered by ethnicity or race. Of equal relevance, BRILLIANCE also supports findings that treatment can be guided by either disease activity or VA stabilization. |
| Comparison of Intravitreal Aflibercept and Ranibizumab for Treatment of Myopic Choroidal Neovascularization: One-Year ResultsA Retrospective, Comparative Study | Comparison between aflibercept and ranibizumab for the treatment of mCNV |
Retrospective chart review Aflibercept 2 mg/0.05 ml (n=12) Ranibizumab 0.5mg/0.05 ml (n=18) |
Mean BCVA from baseline to Month 12 (minimum angle of resolution [logMAR]): Aflibercept group: 1.54 ± 0.76 to 0.85 ± 0.61 (P= .024, Wilcoxon signed rank test) Ranibizumab group: 1.14 ± 0.90 to 1.04 ± 0.93 (P= .345, Wilcoxon signed rank test) Superiority of aflibercept over ranibizumab in means of visual gain at final (12-month) visit (0.69 vs 0.09; P= .006) Both groups showed similar anatomic improvement (mean central macular thickness [CMT] reduction, intraretinal fluid improvement, resolution of subretinal fluid). [Erden.JOphthal.2019] 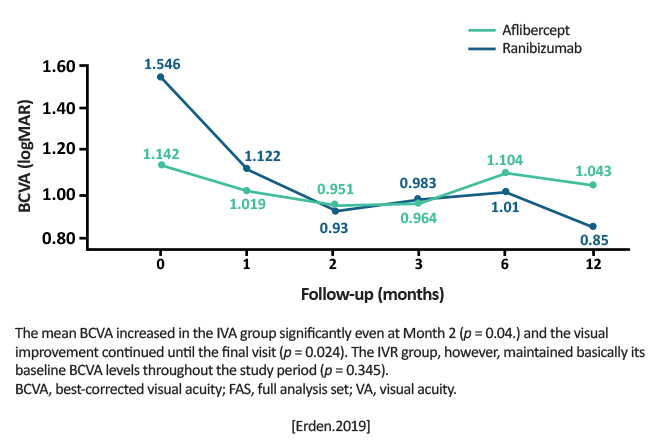
|
Aflibercept proved itself more effective (especially in visual gains) but prospective, randomized clinical trials are warranted to substantiate the claim. [Erden.JOphthal.2019] | First real-world study to compare aflibercept and ranibizumab. The retrospective nature comes with its own set of limitations, but these limited outcomes suggest aflibercept may be more effective than ranibizumab. |
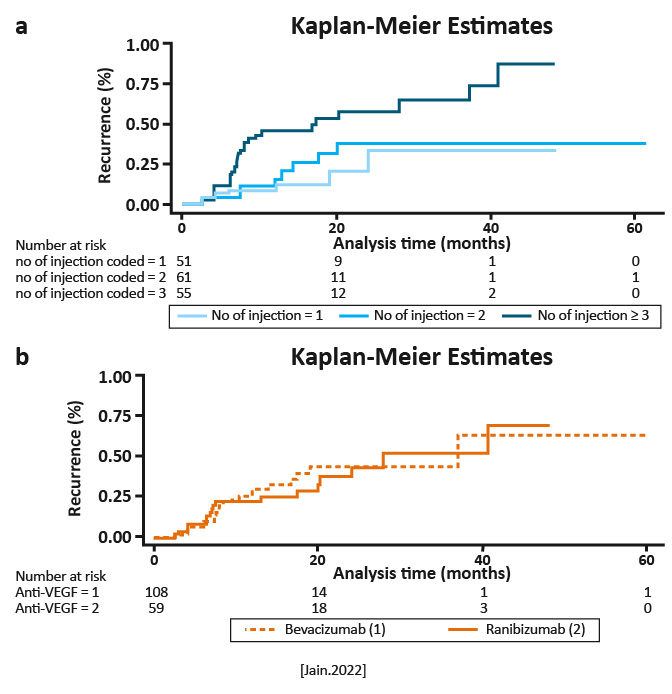
| Incidence, Predictors and Re-treatment Outcomes of Recurrent Myopic Choroidal Neo-vascularization | In this retrospective, consecutive, observational series, to evaluate the incidence, possible predictors, and retreatment outcomes of eyes in patients diagnosed with nave myopic choroidal neovascularization (mCNV) treated with ranibizumab or bevacizumab. | Of 167 patients, 59 eyes were treated with intravitreal ranibizumab; 108 eyes were treated with bevacizumab monotherapy. |
Kaplan-Meier survival analysis showed the risk of recurrence was 8%, 26% and, 33.6% at 6, 12 and 18 months, respectively. Age (P= .511), gender (P=.218), spherical equivalent (P=.092), anti-VEGF (P= .629) and baseline best corrected visual acuity (P= .519) did not influence recurrence. The number of injections administered to control the disease in the first episode was the only significant predictor of recurrence of mCNV.
|
Eyes requiring a greater number of injections for disease control in first episode are at risk for early mCNV recurrence. However, recurrence does not adversely affect visual outcome, if treated adequately. [Jain. PLOSOne.2022] | The risk of recurrence in mCNV is high. The number of injections needed to stabilize initial disease activity predicted recurrence, and eyes requiring three or more injections for initial stabilization specifically are at risk for early recurrence. However, recurrence does not alter the final visual outcome if treated appropriately. [Jain. PLOSOne.2022] |
| Myopic Choroidal Neovascularization: A 10-Year Follow-Up | VA during the 10 years after mCNV onset |
Retrospective clinical study Inclusion criteria for this study were: (1) refractive error of -8 diopters (D) or more; (2) fundus changes typical of pathologic myopia: chorioretinal atrophy, lacquer cracks, atrophic patches; (3) presentation within 6 months of onset of symptoms, that is, loss of visual acuity, metamorphopsia, or both; (4) fluorescein angiographic documentation of macular CNV; and (5) minimum follow-up of 10 years. |
Initial visit: Better than 20/40: 22.2% 20/40-20/200: 48.2% Worse than 20/200: 29.6% 3-year: Better than 20/40: 18.5% 20/40-20/200: 38.0% Worse than 20/200: 44.5% 5-year: Better than 20/40: 3.7% 20/40-20/200: 7.4% Worse than 20/200: 88.9% Final visit: Better than 20/40: 3.7% 20/40-20/200: 0% Worse than 20/200: 96.3% Anatomic outcomes: |
Almost all eyes with mCNV had vision decrease to 20/200 or worse within 10 years of initial visit. [Yoshida.Ophthalmology.2003] | Emphasizes that the natural course of the disease has an extremely poor visual prognosis. This analysis did not evaluate treatment effects. |
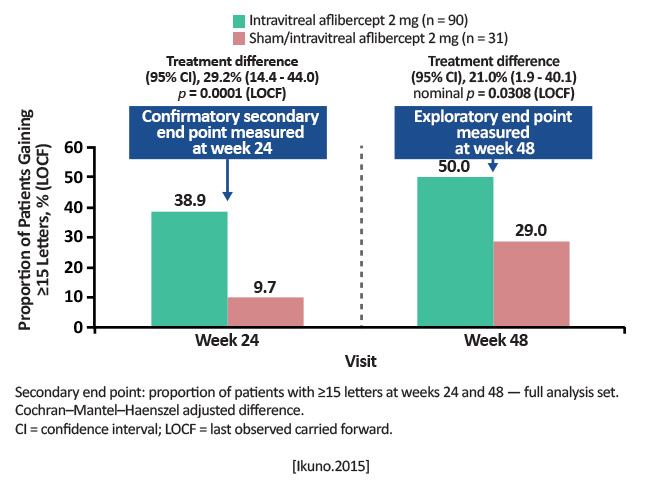
| MYRROR (Intravitreal Aflibercept Injection in Patients With Myopic Choroidal Neovascularization: The MYRROR Study) | Comparison between aflibercept 2 mg and sham in mean change in BCVA from baseline to Week 24 for patients with mCNV (High myopia was defined as 6.0 D or axial length of 26.5 mm) |
Randomized 3:1 Aflibercept 2 mg (n=91) Sham/aflibercept (n=31) Retreatment was allowed if VA decreased by 5 letters, if central retinal thickness (CRT) increased >50 µm from the previous evaluation, or if the investigator deemed it necessary. At Week 24, sham patients were crossed over to aflibercept. |
At Week 24: Aflibercept group: +12.1 letter gain Sham: -2 letter loss (P< .0001). At Week 48: Anatomic outcomes improved in parallel with visual gains. |
Intravitreal aflibercept 2 mg was shown to be a well-tolerated and effective treatment for patients with myopic CNV. Visual improvements and anatomic benefits were maintained and extended from Week 24 to Week 48.
[Ikuno.Ophthalmology.2015] |
Aflibercept is a useful anti-VEGF for mCNV; this study added that patients may only need a limited number of injections early in the course of treatment. Adds to the literature that anti-VEGFs should be considered first-line treatment for mCNV. |
| The Prevalence of Myopic Choroidal Neovascularization in the United States | Prevalence and population burden of high myopia (HM), progressive high myopia (PHM), and myopic choroidal neovascularization (mCNV) among US adults by using 3 national databases: (1) the National Health and Nutrition Examination Survey (NHANES); (2) the US Population Census; and (3) the American Academy of Ophthalmologys (AAOs) Intelligent Research in Sight (IRIS) Registry |
Retrospective analysis of NHANES 2004-2008 participants with data in the AAOs IRIS Registry 2014 Definitions: HM: Refractive error of 6D in the right eye PHM: HM with the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis of 360.21: Progressive high (degenerative) myopia mCNV: HM with the presence of subretinal/CNV indicated by ICD-9-CM diagnosis of 362.16: Retinal neovascularization: not otherwise specified (NOS) |
Prevalence rates in 2014 for: HM: 3.92% [95% confidence interval [CI}, 2.82-5.60]; affecting 9.6 million adults PHM: 0.33% [95% CI, 0.21-0.55]; affecting 818,000 adults mCNV: 0.017% [95% CI, 0.010-0.030]; affecting 41,000 adults [Willis.Ophthalmology.2016] 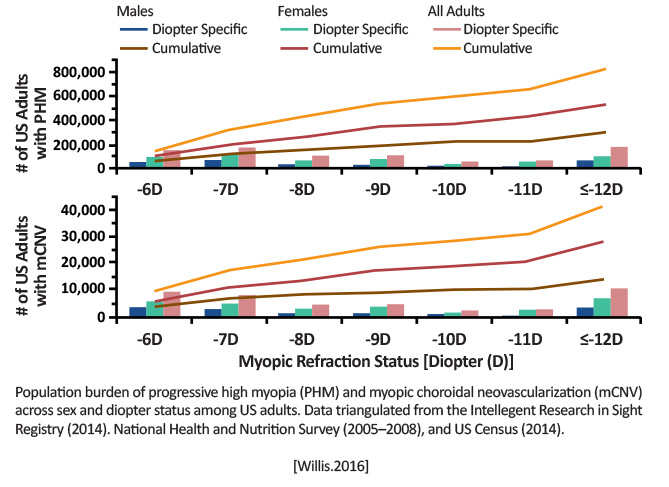
|
mCNV seems to be a rare disease, while HM and PHM pose a large burden among adults in the United States. [Willis.Ophthalmology.2016] |
Outside the United States, multiple studies have shown prevalence of mCNV to be <1% (exception being among Asian patients). The IRIS data confirmed women were more likely to be affected by mCNV than men. [Willis.Ophthalmology.2016] There has been an inconsistency in how PHM and mCNV are diagnosed/recorded. Relating data from both the IRIS Registry and NHANES could be a novel way to assess prevalence (although this is more for researchers than clinicians). |
|
RADIANCE (A Randomized Controlled Study of Ranibizumab in Patients With Choroidal Neovascularization Secondary to Pathologic Myopia) |
Efficacy and safety of 2 different dosing regimens of 0.5 mg ranibizumab compared to verteporfin PDT (vPDT) in patients with visual impairment due to choroidal neovascularization (CNV) secondary to pathologic myopia (PM) Primary outcome: the superiority of ranibizumab 0.5 mg treatment, guided by VA stabilization (Group 1) and/or disease activity (Group 2) criteria, over vPDT (Group 3) at Month 3 |
Group 1 (n=106): ranibizumab on Day 1, Month 1, and thereafter as needed guided by VA stabilization Group 2 (n=116): ranibizumab on Day 1 and thereafter as needed guided by disease activity criteria Group 3 (n=55): vPDT on Day 1 and disease activity treated with ranibizumab or vPDT at investigators™ discretion from Month 3 [Wolf.Ophthalmology.2014] |
BCVA change Month 1 to Month 3: Group 1: +10.5 Group 2: +10.6 Group 3: +2.2 (P< .0001) Mean BCVA change baseline to Month 12: Group 1: +13.8 Group 2: +14.4 Group 3: +9.3 Anatomic outcomes mirrored overall treatment effect of improved BCVA. [Wolf.Ophthalmology.2014] |
The 12-month safety results from the RADIANCE study corroborate the safety findings of the (phase 2, single arm) REPAIR study. Also, the overall safety of ranibizumab treatment in patients with myopic CNV was comparable to the previously established safety profile of ranibizumab in other indications, such as neovascular age-related macular degeneration (AMD), diabetic macular edema, and retinal vein occlusion. [Wolf.Ophthalmology.2014] |
Ranibizumab is a more effective treatment for those with mCNV than vPDT (more than 10 letters compared to 2 letters) up to Month 12. Patients who started on vPDT and switched to ranibizumab after Month 3 also showed gains through Month 12. |
| REPAIR (Ranibizumab in Myopic Choroidal Neovascularization: The 12-Month Results From the REPAIR Study) | Mean change in BCVA score in treatment-nave patients with mCNV treated with intravitreal ranibizumab |
Phase 2, prospective, multicenter study No comparator |
Mean change in BCVA from baseline to Month 12: 13.8±14.0 letters (95% CI, 10.2-17.3) P< .001 Most improvement seen at Month 1: mean +8.7 letters Overall: 15 letters: 24 patients (36.9%) 10 letters: 50.8% Loss of 15 letters: 1 patient (1.5%) Anatomic improvements mimicked visual gains (mean central macular thickness reduction of 135 m from baseline to Month 12; greatest improvement at Month 1) [Tufail.Ophthalmology.2013] |
At the time, REPAIR was the largest, multicenter, prospective study on the treatment of mCNV with ranibizumab. Authors suggested results supported first-line therapy with ranibizumab in treatment-nve myopic CNV. [Tufail.Ophthalmology.2013] |
Patient-reported outcomes were significantly improved at 12 months, and provided the groundwork for the phase 3 RADIANCE study. |
| VIP (Photodynamic Therapy of Subfoveal Choroidal Neovascularization in Pathologic Myopia With Verteporfin: 1-Year Results of a Randomized Clinical Trial VIP Report No. 1) | Proportion of eyes at the follow-up examination 12 months after study entry with fewer than 8 letters (approximately 1.5 lines) of visual acuity lost, adhering to an intent-to-treat analysis |
PDT arm: 81 eyes Placebo: 39 eyes |
Vision remaining stable (loss of <8 letters) at Month 3: vPDT: 77% (n=62) Placebo: 56% (n=22) P= .02 At Month 12: vPDT: 72% (n=55) Placebo: 44% (n=17) P< .01 [VIP Study Group.Ophthalmology. 2001] 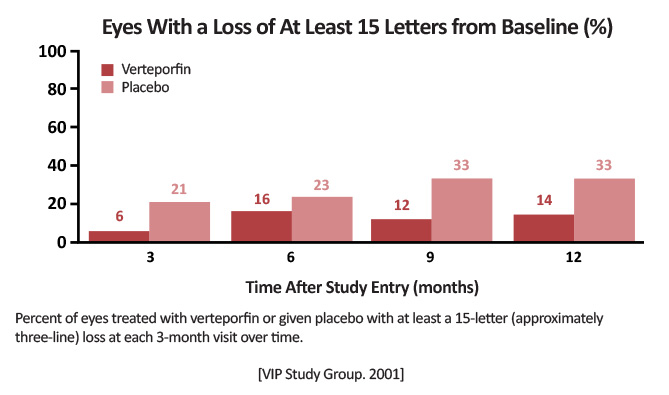
|
Verteporfin PDT has a significant treatment benefit for subfoveal CNV caused by pathologic myopia as well as for subfoveal CNV that is predominantly classic in AMD. Authors recommended vPDT as treatment for these patients. [VIP Study Group.Ophthalmology. 2001] |
First study that showed efficacy of vPDT in stabilizing or improving vision in patients with subfoveal CNV from pathologic myopia. Led to vPDT becoming standard of care until the introduction of anti-VEGF agents. |