| Clinical study "common" name (formal title) | Number of enrollees (per protocol [PP]/intent-to-treat [ITT]) | Funding source (pharmaceutical company/National Institutes of Health [NIH]) | Year completed | Primary publication | Full study treatment protocol |
|---|---|---|---|---|---|
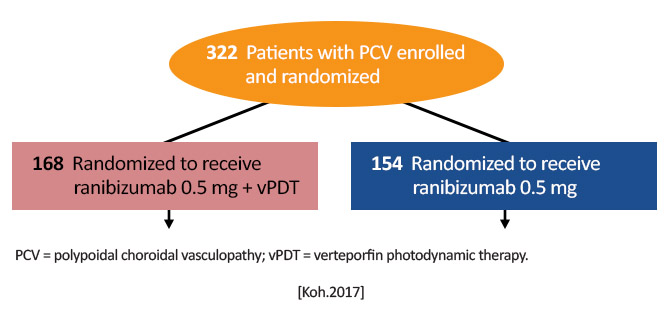
| EVEREST II (Efficacy and Safety of Ranibizumab With or Without Verteporfin Photodynamic Therapy for Polypoidal Choroidal Vasculopathy: A Randomized Clinical Trial) | 322 patients from Hong Kong, Japan, South Korea, Malaysia, Singapore, Taiwan, and Thailand | Novartis NCT01846273 | 2017 |
EVEREST II (Visual Outcome in Patients With Symptomatic Macular Polypoidal Choroidal Vasculopathy [PCV] Treated With Either Ranibizumab as Monotherapy or Combined With Verteporfin Photodynamic Therapy)
Koh A, et al. Efficacy and safety of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: a randomized clinical trial. JAMA Ophthalmol. 2017;135(11): 1206-1213.
https://jamanetwork.com/journals/jamaophthalmology/fullarticle/2768203 |
|