| Clinical Study “Common” Name (formal title) | Study design | Treatment arms | Primary outcomes | Results | Author conclusions | "Real-world" impact |
|---|---|---|---|---|---|---|
| Dexamethasone Intravitreal Implant for Noninfectious Intermediate or Posterior Uveitis | Prospective, multicenter, masked, randomized, parallel-group, sham-controlled trial | A single treatment with a 0.7-mg DEX implant (n = 77) A single treatment with a 0.35-mg DEX implant (n = 76) A sham procedure (n = 76) | In this 26-week trial, the proportion of eyes with a 0.7- or 0.35-mg dexamethasone intravitreal implant that had a vitreous haze score of 0 at week 8. All eyes had noninfectious intermediate or posterior uveitis. |
Proportion of eyes with a vitreous haze score of 0 at week 8: 47% with the 0.7-mg DEX implant. 36% with the 0.35-mg DEX implant. 12% with the sham. A gain of 15 letters or more from baseline best-corrected visual acuity was observed significantly more in the DEX implant groups than the sham group. Benefits persisted through week 26. |
In patients with noninfectious intermediate or posterior uveitis, the DEX implant significantly improved intraocular inflammation and visual acuity and persisted for 6 months. |
The dexamethasone intravitreal implant may be used safely and effectively to treat intermediate and posterior uveitis.
The 0.7-mg implant demonstrated better efficacy with similar safety when compared with the 0.35-mg implant. [Lowder.Arch Ophthalmol.2011] |
|
FAST (Effect of Corticosteroid-Sparing Treatment With Mycophenolate Mofetil vs Methotrexate on Inflammation in Patients With Uveitis) |
Multicenter, randomized, parallel, observer-masked trial |
Oral methotrexate (25 mg) weekly (n = 107) or oral mycophenolate mofetil (3 g) daily (n = 109) |
To compare methotrexate and mycophenolate to achieve corticosteroid-sparing control of noninfectious intermediate uveitis, posterior uveitis, and panuveitis and treatment success at 6 months. Treatment success was defined as (a) control of inflammation in both eyes, (b) no more than 7.5 mg of prednisolone daily, (c) less than or equal to 2 drops of prednisolone acetate 1%, and (d) no treatment failure due to safety or intolerability. |
In the methotrexate group, 66.7% of patients achieved corticosteroid-sparing control. In the mycophenolate group, 57.1% achieved corticosteroid-sparing control (P= .20). The difference was not statistically significant. Among patients with posterior uveitis, treatment success was achieved among 74.4% in the methotrexate group versus 55.3% in the mycophenolate group (P= .02 ). 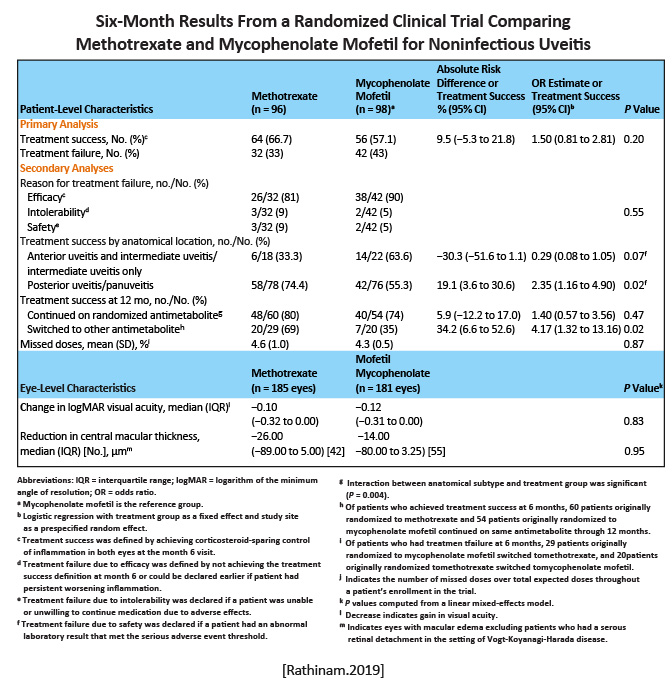
|
The use of mycophenolate mofetil compared with methotrexate as a first-line corticosteroid-sparing treatment did not result in superior control of inflammation. In patients with posterior uveitis and panuveitis, who often have more vision complications than other uveitis patients, methotrexate may be a reasonable first-line choice. [Rathinam.JAMA. 2019] |
The findings provided scientific justification that mycophenolate mofetil is not more effective than methotrexate as a corticosteroid-sparing immunosuppressive therapy for uveitis. [Rathinam.JAMA.2019] |
|
MUST Randomized Comparison of Systemic Anti-inflammatory Therapy Versus Fluocinolone Acetonide Implant for Intermediate, Posterior and Panuveitis: The Multicenter Uveitis Steroid Treatment Trial |
Randomized, partially masked, 23-center parallel treatment comparative effectiveness superiority trial | 1:1 randomization (n = 255) to fluocinolone acetonide implant therapy versus systemic corticosteroids plus immunosuppression. | Change in best-corrected visual acuity at 24 months from baseline among patients with noninfectious intermediate, posterior, or panuveitis receiving systemic versus implant therapy. |
The implant group had a +6.0 letters improvement in visual acuity versus +3.2 letters for the systemic therapy group.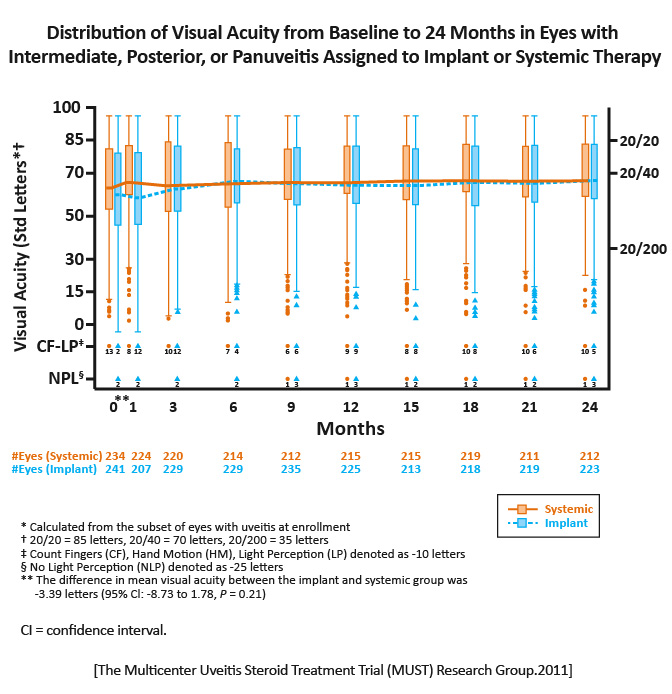
|
In each treatment group, the mean visual acuity improved over 24 months. Neither approach was superior. Both approaches usually succeeded in controlling ocular inflammation, but implant therapy gained control faster and more often. [MUST Trial Research Group.Ophthalmology.2011] |
Specific advantages and disadvantages identified should dictate selection between the alternative treatments in consideration of individual patients particular circumstances. [MUST Trial Research Group.Ophthalmology.2011] |
|
MUST Follow-up Longitudinal Vision-Related Quality of Life for Patients with Noninfectious Uveitis Treated with Fluocinolone Acetonide Implant or Systemic Corticosteroid Therapy |
Data from the NEI-VFQ-25 for the first 3 years after randomization (129 for implant therapy; 126 for systemic therapies). | National Eye Institute Visual Functioning Questionnaire-25 (NEI-VFQ-25) composite score over 3 years after randomization in the MUST Trial. |
Individuals in both groups showed similar improvement in NEI-VFQ-25 scores after 3 years (implant group, 11.9 points; systemic, 9.0 points).
There was substantial improvement in the implant group in the first 6 months followed by stable scores. In the systemic group, there was steady improvement. |
Both treatment groups demonstrated significant improvements in NEI-VFQ-25 scores, with more immediate improvement in the implant group.
Poorer visual function was associated significantly with initial differences in NEI-VFQ-25 scores, yet only individuals in the implant group with poor visual acuity were able to overcome their initial deficits by the end of 3 years. [Sugar.Ophthalmology.2017] |
Both treatments for noninfectious uveitis resulted in significant improvements in NEI-VFQ-25 composite scores, but longitudinal patterns differed. [Sugar.Ophthalmology.2017] |
|
|
POINT Periocular Triamcinolone Versus Intravitreal Triamcinolone Versus Intravitreal Dexamethasone Implant for the Treatment of Uveitic Macular Edema: The PeriOcular versus INTravitreal Corticosteroids for Uveitic Macular Edema (POINT) Trial |
Multicenter, randomized, parallel-treatment, comparative trial | 1:1:1 randomization to receive one of the three therapies: periocular triamcinolone acetonide (73 eyes), intravitreal triamcinolone acetonide (79 eyes), and the intravitreal dexamethasone implant (78 eyes). | Proportion of baseline central subfield thickness at 8 weeks assessed with optical coherence tomography by masked readers when comparing the effectiveness of three regional corticosteroid injections for uveitic macular edema: periocular triamcinolone acetonide, intravitreal triamcinolone acetonide, and the intravitreal dexamethasone implant. |
All treatment groups had improved central subfield thickness at 8 weeks relative to baseline. There were reductions of 23%, 39%, and 46% for periocular triamcinolone acetonide, intravitreal triamcinolone acetonide, and the intravitreal dexamethasone implant, respectively.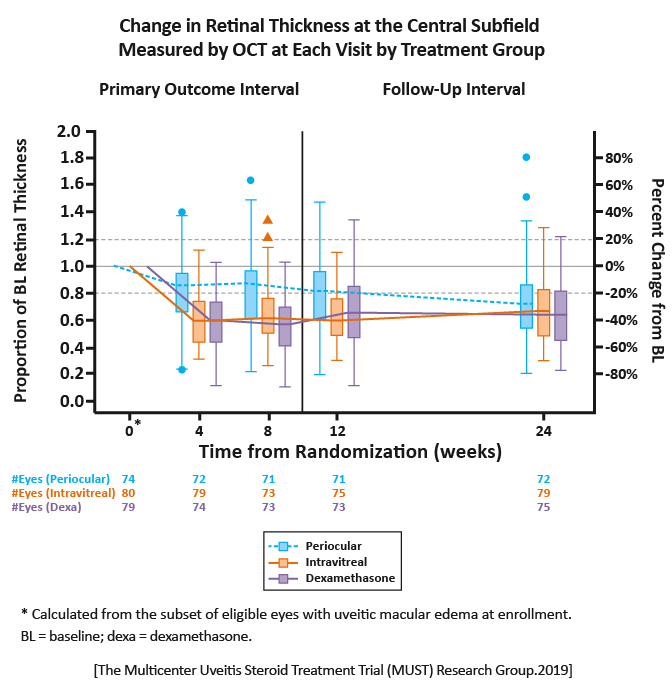
|
Both intravitreal triamcinolone acetonide and the dexamethasone implant were superior to periocular triamcinolone acetonide for the treatment of uveitic macular edema with modestly greater rates of mild IOP elevation. Intravitreal therapy may be the preferred initial therapy for uveitic macular edema. [MUST Research Group.Ophthalmology.2019] |
This was the first head-to-head comparison of these three common therapies.
Because uveitic macular edema represents a significant cause of ocular morbidity and remains a leading cause of visual impairment, comparative data from a randomized trial with an objective and masked primary outcome measure provides insight into the regional corticosteroid that effectively balances efficacy and safety for this patient group. [MUST Research Group.Ophthalmology.2019] |
| Randomized trial | 1:1 randomization to receive adalimumab (a loading dose of 80 mg followed by a 40-mg dose every 2 weeks) or matched placebo. All patients received a mandatory prednisone burst followed by prednisone tapering over 15 weeks. | Time to treatment failure occurring at or after week 6 among noninfectious intermediate uveitis, posterior uveitis, or panuveitis patients receiving adalimumab or placebo. |
The median time to treatment failure was 24 weeks in the adalimumab group and 13 weeks in the placebo group. Among the 217 patients in the intention-to-treat population, those receiving adalimumab were less likely than those in the placebo group to have treatment failure. 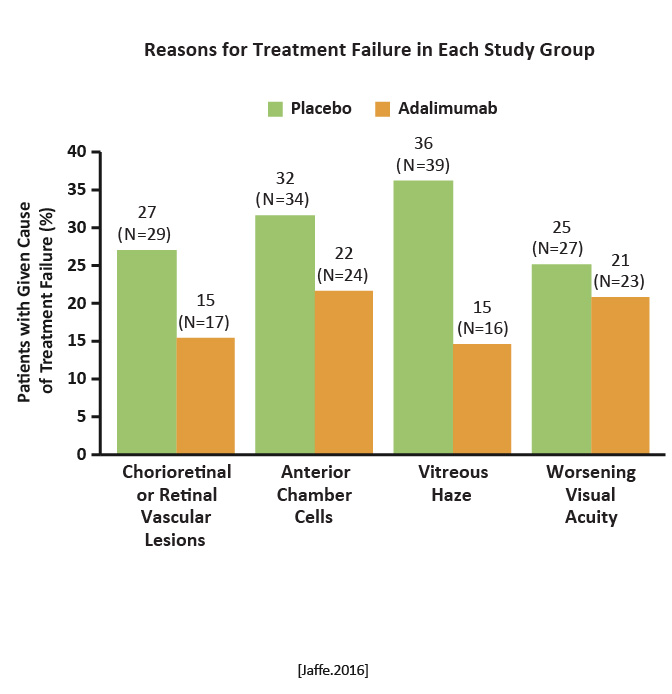 Reason for treatment failure in each study group Reason for treatment failure in each study group
|
Adalimumab was associated with a lower risk of uveitic flare or visual impairment and with more adverse and serious adverse events than placebo. [Jaffe.NEJM.2016] |
There are few randomized controlled trials of immunosuppressive therapies. This studys design, large patient population, and diversity of uveitis diagnoses, among other factors, advance research in this area. [Jaffe.NEJM.2016] |
VISUAL 1 Adalimumab in Patients with Active Noninfectious Uveitis |