| Clinical Study “Common” Name (formal title) | Study design | Treatment arms | Primary outcomes | Results | Author conclusions | "Real-world" impact |
|---|---|---|---|---|---|---|
|
CARE-ROP [Multicenter Randomized Double Masked Parallel Design Exploratory Study to Assess Safety and Efficacy of Two Different Doses of Intravitreal Anti-VEGF Treatment With Ranibizumab (0.12 mg vs. 0.20 mg) in Infants With Retinopathy of Prematurity (ROP)] |
Randomized, multicenter, prospective, double-blind, 2-arm, parallel-group, phase 2, investigator-initiated trial of lower doses of ranibizumab (0.12 mg and 0.20 mg) |
Gestational age ≤ 25 weeks (n = 11): 0.12 mg ranibizumab (n = 6) 0.20 mg ranibizumab (n = 5) Gestational age > 25 weeks (n = 8): 0.12 mg ranibizumab (n = 4) 0.20 mg ranibizumab (n = 4) |
The primary endpoint was the number of infants who did not require rescue therapy at 24 weeks. Key secondary endpoints included time-to-event analyses, progression of physiologic vascularization, and plasma VEGF levels. (Rescue therapy was defined as the need for either laser photocoagulation or 0.2-mg ranibizumab reinjection within 4 weeks after study treatment or the need for laser treatment at any other timepoint.) |
Number of patients without rescue therapy up to week 24 (3 patients died before reaching study end point; deaths were not attributable to study drug): Ranibizumab 0.12 mg (n = 9) (8 without rescue therapy, 88.9%) Ranibizumab 0.20 mg (n = 7) (6 without rescue therapy, 85.7%) [Stahl.JAMA Peds.2018] No infant with GA > 25 weeks needed rescue therapy. Ranibizumab 0.12 mg: 12/20 eyes (60%) had no ROP at final visit Ranibizumab 0.20 mg: 10/18 eyes (55.6%) had no ROP at final visit. [Stahl.JAMA Peds.2018] |
Control of ROP without need for rescue therapy was achieved in 14 of 16 surviving infants (87.5%) (30 of 32 eyes [93.8%]), and there was no significant difference between the two dose groups regarding primary end point outcomes. [Stahl.JAMA Peds.2018] |
This is the first study comparing ranibizumab doses that are lower than 50% of adult doses to treat ROP. The study showed the lower of these two doses (0.12 mg) is as effective as the 0.20 dose. Ranibizumab 0.20 mg is approved in Europe in preterm infants for the treatment of ROP with zone I (stage 1+, 2+, 3 or 3+), zone I (stage 3+) or aggressive posterior ROP disease. This study supports using an even lower dose with similar results. |
| Clinical Risk Factors for Retinopathy of Prematurity Reactivation after Intravitreal Anti-Vascular Endothelial Growth Factor Injection | Retrospective evaluation of all premature infants admitted to Chang Gung Memorial Hospital (Taiwan) between Jan. 2017 and Dec. 2022 who received intravitreal injections of anti-VEGF agents (aflibercept, bevacizumab, or ranibizumab) for the treatment of ROP. |
Two arms: ROP reactivation after intravitreal injection therapy No ROP reactivation after intravitreal injection therapy (ROP reactivation was defined as the presence of new lines or ridges that have reached stage 3 (reactivated stage 3) with plus disease, or recurrent vascular dilation and/or tortuosity, or the presence of new extraretinal vessels, according to international classification of ROP, 3rd edition). |
The primary outcome was the rate of ROP reactivation. |
ROP reactivation: Aflibercept 0.8-2.0 mg (n = 72): 10 (13.9%) had reactivation Bevacizumab 0.625 mg (n = 133): 8 (6%) had reactivation Ranibizumab 0.25 mg (n = 18): 4 (22.2%) had reactivation Differences when compared to bevacizumab was not statistically significant for aflibercept (P = .064) but was statistically significant for ranibizumab (P = .025) 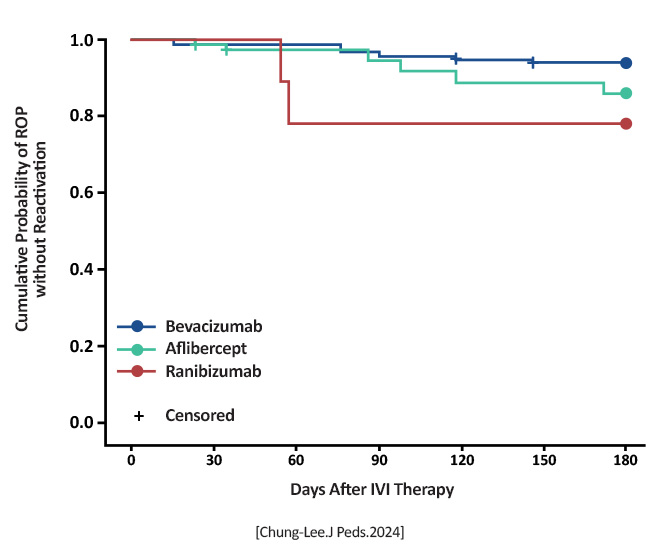 [Chung-Lee.J Peds.2024] |
All anti-VEGF agents carry a risk of ROP reactivation (occurring in about 10% of infants), with the risk being higher with ranibizumab 0.25 mg than with bevacizumab 0.625 mg [Chung-Lee.J Peds.2024] |
Adds to the literature advocating lower-dose anti-VEGF treatments for ROP. The ranibizumab dose was higher than in other studies (CARE-ROP), which may explain the higher reactivation rate. |
|
ETROP Final Results of the Early Treatment of Retinopathy of Prematurity (ETROP) Trial |
Randomized trial | Early retinal ablative treatment (n = 317) and the fellow eye managed conventionally (control eye) in infants with bilateral high-risk prethreshold ROP. For asymmetric cases (n = 84), the eye with high-risk prethreshold ROP was randomized to early retinal ablative treatment or to conventional management. | Visual acuity as assessed by masked testers using the Teller acuity card procedure. Structural examinations were performed at 6 and 9 months corrected age. | A reduction in unfavorable visual acuity outcomes occurred with earlier treatment, from 19.8% to 14.3%. Unfavorable structural outcomes were reduced from 15.6% to 9.0% at 9 months. | Early treatment of high-risk prethreshold ROP significantly reduced unfavorable outcomes in both primary and secondary/structural measures. [Good WV.Trans Am Ophthalmol Soc.2004] | It is possible to identify characteristics of ROP that predict which eyes are most likely to benefit from early peripheral retinal ablation. The clinical algorithm developed with this study provided treatment guidance for peripheral retinal ablation for infants with Type 1 and Type 2 ROP. [Good WV.Trans Am Ophthalmol Soc.2004] |
|
CRYO-ROP Multicenter trial of cryotherapy for retinopathy of prematurity: preliminary results. Cryotherapy for Retinopathy of Prematurity Cooperative Group |
Multicenter randomized trial |
1988: One eye of each patient with symmetrical ROP was randomly selected for transscleral cryotherapy.
2005: Report of the ocular structure and visual acuity outcomes at age 15 years, and the incidence of retinal detachment between 10 and 15 years of age, for patients in the CRYO-ROP trial.
|
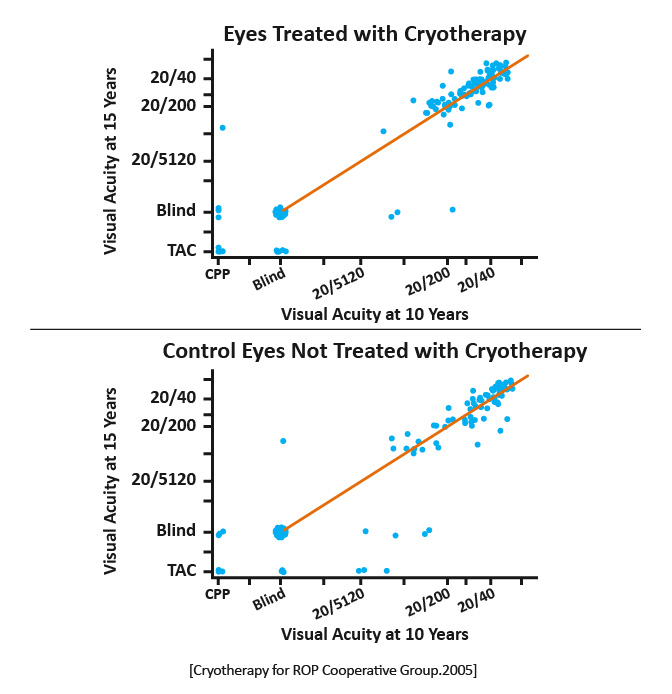
Structural and functional outcomes of transscleral cryotherapy for the treatment of ROP. |
1988: An unfavorable outcome was significantly less frequent in the eyes undergoing cryotherapy (21.8%) compared with the untreated eyes (43%).
2005: Thirty percent of treated eyes and 51.9% of control eyes had unfavorable structural outcomes.
|
1988: These data support the efficacy of cryotherapy in reducing by approximately one half the risk of unfavorable retinal outcome from threshold ROP. [Cryotherapy for ROP Cooperative Group.Pediatrics.1988] 2005: The benefit of cryotherapy for treatment of threshold ROP, for both structure and visual function, was maintained across 15 years of follow-up. New retinal detachments, even in eyes with relatively good structural findings at age 10 years, suggest value in long-term, regular follow-up of eyes that experience threshold ROP. [Cryotherapy for ROP Cooperative Group.ArchOphthalmol.2005] | 1988: Cryotherapy is recommended for at least one eye in symmetric ROP cases. For the fellow eye and for the single eye in asymmetric cases, the surgeon should use clinical judgment. [Cryotherapy for ROP Cooperative Group.Pediatrics.1988] 2005: Results from CRYO-ROP examinations conducted at 3.5, 5.5, and 10 years indicated that eyes that were saved from blindness by cryotherapy developed visual acuity that was better than 20/200 but worse than the normal range for age. [Cryotherapy for ROP Cooperative Group.ArchOphthalmol.2005] |
|
BEAT-ROP Efficacy of Intravitreal Bevacizumab for Stage 3+ Retinopathy of Prematurity |
Prospective, controlled, randomized, stratified, multicenter trial | Infants (n =150, with 143 infants [286 eyes] completing the trial) were randomized to receive intravitreal bevacizumab (0.625 mg in 0.025 ml of solution) or conventional laser therapy, bilaterally. | Recurrence of retinopathy of prematurity in one or both eyes requiring retreatment before 54 weeks' postmenstrual age. |
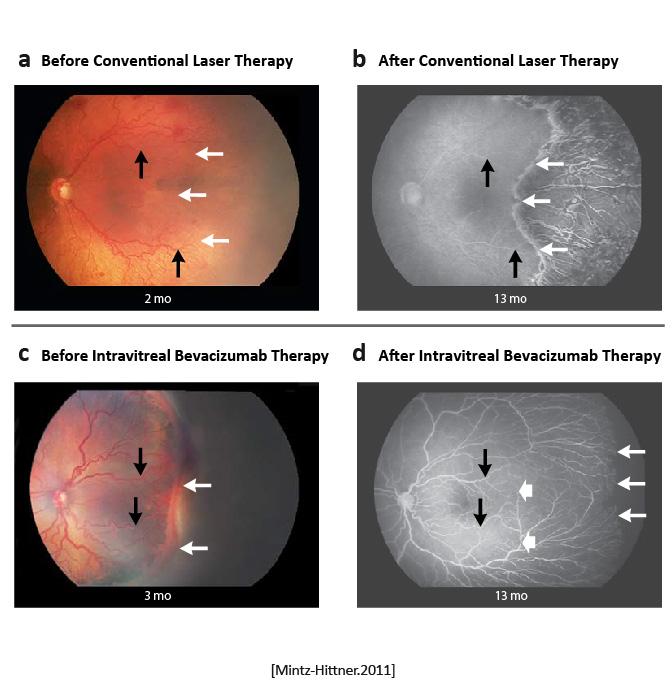
Retinopathy of prematurity recurred in four infants in the bevacizumab group (6 of 140 eyes); ROP recurred in 19 infants in the laser-therapy group (32 of 146 eyes. A significant treatment effect was found for zone I ROP but not for zone II disease.
|
Intravitreal bevacizumab monotherapy, as compared with conventional laser therapy, in infants with stage 3+ ROP showed a significant benefit for zone I but not zone II disease. Development of peripheral retinal vessels continued after treatment with intravitreal bevacizumab, but conventional laser therapy led to permanent destruction of the peripheral retina. [Mintz-Hittner.NEJM.2011] | Bevacizumab is an inexpensive drug that can be rapidly administered at the bedside by any ophthalmologist. In contrast, conventional laser therapy is laborious and requires special training to administer, as well as expensive equipment, endotracheal intubation, and a location designated for the use of lasers. However, caution must be taken when administering intravitreal bevacizumab to neonates. [Mintz-Hittner.NEJM.2011] |
|
RAINBOW Ranibizumab Versus Laser for the Treatment of Very Low Birthweight Infants With Retinopathy of Prematurity (RAINBOW): An Open-Label Randomised Controlled Trial |
Randomized, open-label, superiority multicenter, three-arm, parallel group trial | Equal randomization (1:1:1) to receive a single bilateral intravitreal dose of ranibizumab 0.2 mg, ranibizumab 0.1 mg, or laser therapy. | Survival with no active retinopathy, no unfavorable structural outcomes, or need for a different treatment modality at or before 24 weeks. |
Treatment success occurred in 56 (80%) of 70 infants receiving ranibizumab 0.2 mg compared with 57 (75%) of 76 infants receiving ranibizumab 0.1 mg and 45 (66%) of 68 infants after laser therapy.
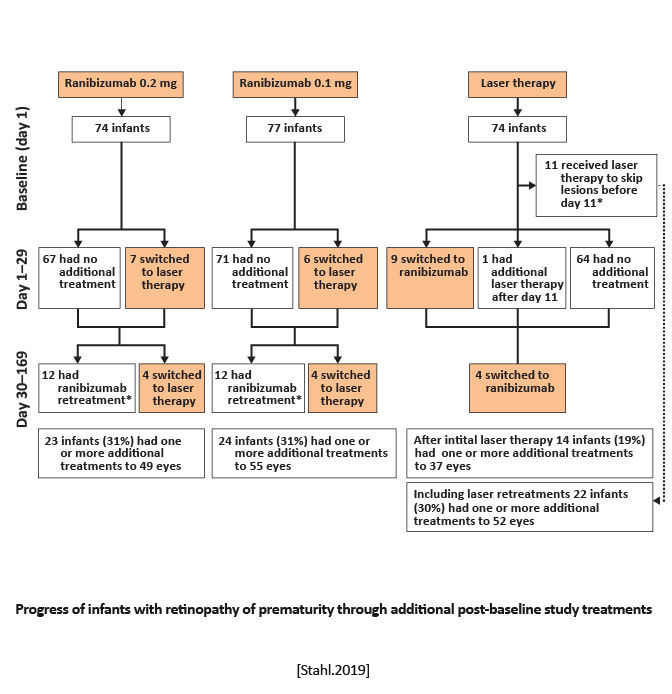
|
Ranibizumab 0.2 mg was as effective and safe in the treatment of active ROP as laser therapy, might be superior, and was associated with better short-term outcomes. [Stahl.Lancet.2019] | Intravitreal injection of ranibizumab can be considered as a novel treatment for ROP. However, disease recurrence must be monitored closely because it is common with anti-VEGF agents and can occur later compared with laser therapy. [Stahl.Lancet.2019] |
|
FIREFLEYE Effect of Intravitreal Aflibercept vs Laser Photocoagulation on Treatment Success of Retinopathy of Prematurity: The FIREFLEYE Randomized Clinical Trial |
Noninferiority randomized trial | Infants were randomized 2:1 to receive a 0.4-mg dose of intravitreal aflibercept (n75) or laser photocoagulation (n43) at baseline. Additional treatment was allowed as prespecified. | Proportion of infants without active retinopathy of prematurity and unfavorable structural outcomes at week 24 after starting treatment. |
Treatment success was 85.5% with intravitreal aflibercept vs 82.1% with laser photocoagulation. Rescue treatment was required in 4.8% of eyes in the intravitreal aflibercept group vs 11.1% of eyes in the laser photocoagulation group.
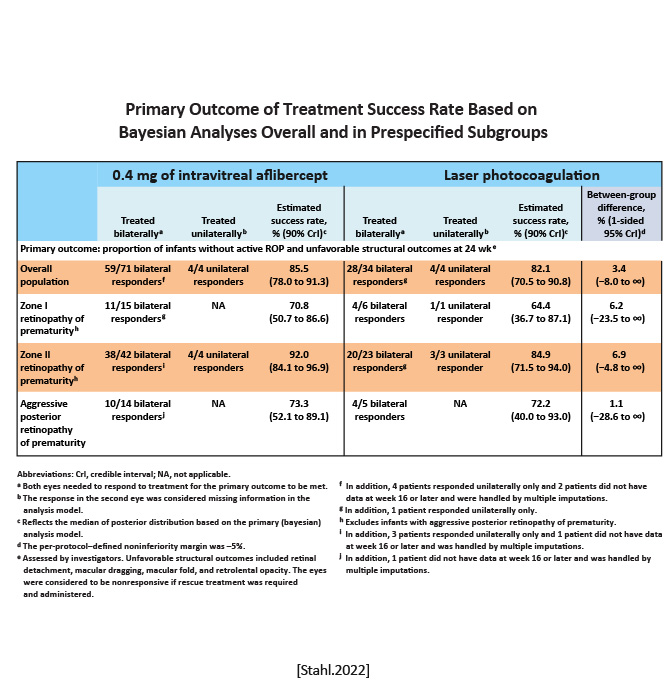
|
Among infants with ROP, intravitreal aflibercept compared with laser photocoagulation did not meet criteria for noninferiority with respect to the primary outcome of the proportion of infants achieving treatment success at week 24. [Stahl.JAMA.2022] | In this noninferiority randomized clinical trial of infants with ROP, intravitreal aflibercept compared with laser photocoagulation did not meet the noninferiority margin of 5% with regard to treatment success. Overall, the study showed a response rate to intravitreal aflibercept within the expected range, considering randomized clinical trials of other anti-VEGF agents for the treatment of ROP. [Stahl.JAMA.2022] |
|
SAFER-ROP: Updated Protocol for Anti-VEGF Injections for Retinopathy of Prematurity |
A retrospective chart review of the SAFER injection protocol:(S)hort needle (4-mm length), (A)ntiseptic/antibiotic (5% to 10% topical betadine), (F)ollowup (48 to 72 hours post-injection), (E)xtra attention to detail (clean environment, injection site 0.75 mm to 1.0 mm posterior to limbus), and (R)echeck (1 to 2 weeks following injection and until mature vascularization or laser). | To describe a safe and dependable protocol for intravitreal injections for the treatment of ROP using the SAFER injection protocol. | No cases of cataract formation, endophthalmitis, or vitreous hemorrhage using this technique were reported. | The SAFER protocol is a safe way to inject anti-vascular endothelial growth factor and to monitor ROP progression following injection. [Beck.Ophthal Surg Lasers Imaging Retina.2020] | The use of a shorter needle in a sterile environment enhances the likelihood of safe drug delivery, but the actual injection is not the only important aspect of the process. Attention to detail and close follow-up are mandatory in order to monitor and treat for infection and to ensure mature vascularization has occurred. Laser should be subsequently performed if vascularization has not occurred by the expected time course. [Beck.Ophthal Surg Lasers Imaging Retina.2020] |