| Clinical Study "Common" Name (formal title) | Study design | Treatment arms | Primary outcomes | Results | Author conclusions | "Real-world" impact |
|---|---|---|---|---|---|---|
|
BEAT-ROP Efficacy of Intravitreal Bevacizumab for Stage 3+ Retinopathy of Prematurity |
Prospective, controlled, randomized, stratified, multicenter trial | Infants (n =150, with 143 infants [286 eyes] completing the trial) were randomized to receive intravitreal bevacizumab (0.625 mg in 0.025 ml of solution) or conventional laser therapy, bilaterally. | Recurrence of retinopathy of prematurity in one or both eyes requiring retreatment before 54 weeks' postmenstrual age. |
Retinopathy of prematurity recurred in four infants in the bevacizumab group (6 of 140 eyes); ROP recurred in 19 infants in the laser-therapy group (32 of 146 eyes. A significant treatment effect was found for zone I ROP but not for zone II disease.
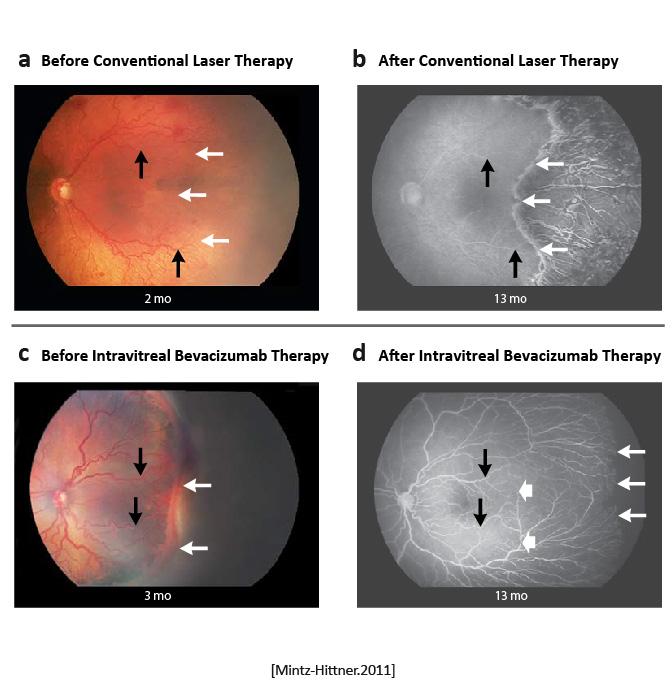
|
Intravitreal bevacizumab monotherapy, as compared with conventional laser therapy, in infants with stage 3+ ROP showed a significant benefit for zone I but not zone II disease. Development of peripheral retinal vessels continued after treatment with intravitreal bevacizumab, but conventional laser therapy led to permanent destruction of the peripheral retina. [Mintz-Hittner.NEJM.2011] | Bevacizumab is an inexpensive drug that can be rapidly administered at the bedside by any ophthalmologist. In contrast, conventional laser therapy is laborious and requires special training to administer, as well as expensive equipment, endotracheal intubation, and a location designated for the use of lasers. However, caution must be taken when administering intravitreal bevacizumab to neonates. [Mintz-Hittner.NEJM.2011] |
|
CARE-ROP [Multicenter Randomized Double Masked Parallel Design Exploratory Study to Assess Safety and Efficacy of Two Different Doses of Intravitreal Anti-VEGF Treatment With Ranibizumab (0.12 mg vs. 0.20 mg) in Infants With Retinopathy of Prematurity (ROP)] |
Randomized, multicenter, prospective, double-blind, 2-arm, parallel-group, phase 2, investigator-initiated trial of lower doses of ranibizumab (0.12 mg and 0.20 mg) |
Gestational age ≤ 25 weeks (n = 11): 0.12 mg ranibizumab (n = 6) 0.20 mg ranibizumab (n = 5) Gestational age > 25 weeks (n = 8): 0.12 mg ranibizumab (n = 4) 0.20 mg ranibizumab (n = 4) |
The primary endpoint was the number of infants who did not require rescue therapy at 24 weeks. Key secondary endpoints included time-to-event analyses, progression of physiologic vascularization, and plasma VEGF levels. (Rescue therapy was defined as the need for either laser photocoagulation or 0.2-mg ranibizumab reinjection within 4 weeks after study treatment or the need for laser treatment at any other timepoint.) |
Number of patients without rescue therapy up to week 24 (3 patients died before reaching study end point; deaths were not attributable to study drug): Ranibizumab 0.12 mg (n = 9) (8 without rescue therapy, 88.9%) Ranibizumab 0.20 mg (n = 7) (6 without rescue therapy, 85.7%) [Stahl.JAMA Peds.2018] No infant with GA > 25 weeks needed rescue therapy. Ranibizumab 0.12 mg: 12/20 eyes (60%) had no ROP at final visit Ranibizumab 0.20 mg: 10/18 eyes (55.6%) had no ROP at final visit. [Stahl.JAMA Peds.2018] |
Control of ROP without need for rescue therapy was achieved in 14 of 16 surviving infants (87.5%) (30 of 32 eyes [93.8%]), and there was no significant difference between the two dose groups regarding primary end point outcomes. [Stahl.JAMA Peds.2018] |
This is the first study comparing ranibizumab doses that are lower than 50% of adult doses to treat ROP. The study showed the lower of these two doses (0.12 mg) is as effective as the 0.20 dose. Ranibizumab 0.20 mg is approved in Europe in preterm infants for the treatment of ROP with zone I (stage 1+, 2+, 3 or 3+), zone I (stage 3+) or aggressive posterior ROP disease. This study supports using an even lower dose with similar results. |
| Clinical Risk Factors for Retinopathy of Prematurity Reactivation after Intravitreal Anti-Vascular Endothelial Growth Factor Injection | Retrospective evaluation of all premature infants admitted to Chang Gung Memorial Hospital (Taiwan) between Jan. 2017 and Dec. 2022 who received intravitreal injections of anti-VEGF agents (aflibercept, bevacizumab, or ranibizumab) for the treatment of ROP. |
Two arms: ROP reactivation after intravitreal injection therapy No ROP reactivation after intravitreal injection therapy (ROP reactivation was defined as the presence of new lines or ridges that have reached stage 3 (reactivated stage 3) with plus disease, or recurrent vascular dilation and/or tortuosity, or the presence of new extraretinal vessels, according to international classification of ROP, 3rd edition). |
The primary outcome was the rate of ROP reactivation. |
ROP reactivation: Aflibercept 0.8-2.0 mg (n = 72): 10 (13.9%) had reactivation Bevacizumab 0.625 mg (n = 133): 8 (6%) had reactivation Ranibizumab 0.25 mg (n = 18): 4 (22.2%) had reactivation Differences when compared to bevacizumab was not statistically significant for aflibercept (P = .064) but was statistically significant for ranibizumab (P = .025) 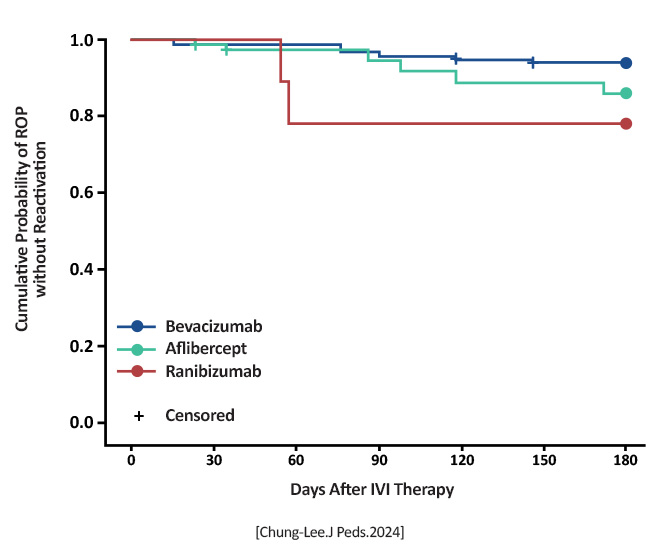 [Chung-Lee.J Peds.2024] |
All anti-VEGF agents carry a risk of ROP reactivation (occurring in about 10% of infants), with the risk being higher with ranibizumab 0.25 mg than with bevacizumab 0.625 mg [Chung-Lee.J Peds.2024] |
Adds to the literature advocating lower-dose anti-VEGF treatments for ROP. The ranibizumab dose was higher than in other studies (CARE-ROP), which may explain the higher reactivation rate. |
| Comparing safety and efficacy of bevacizumab, ranibizumab and ranibizumab biosimilar in retinopathy of prematurity | Retrospective, single center study |
Bevacizumab 0.5 mg (IVB) Ranibizumab 0.2 mg (IVA) Razumab™ biosimilar 0.2 mg (IVR) |
Retreatment rates, time to retreatment, vascularization up to ora, time to vascularization up to ora |
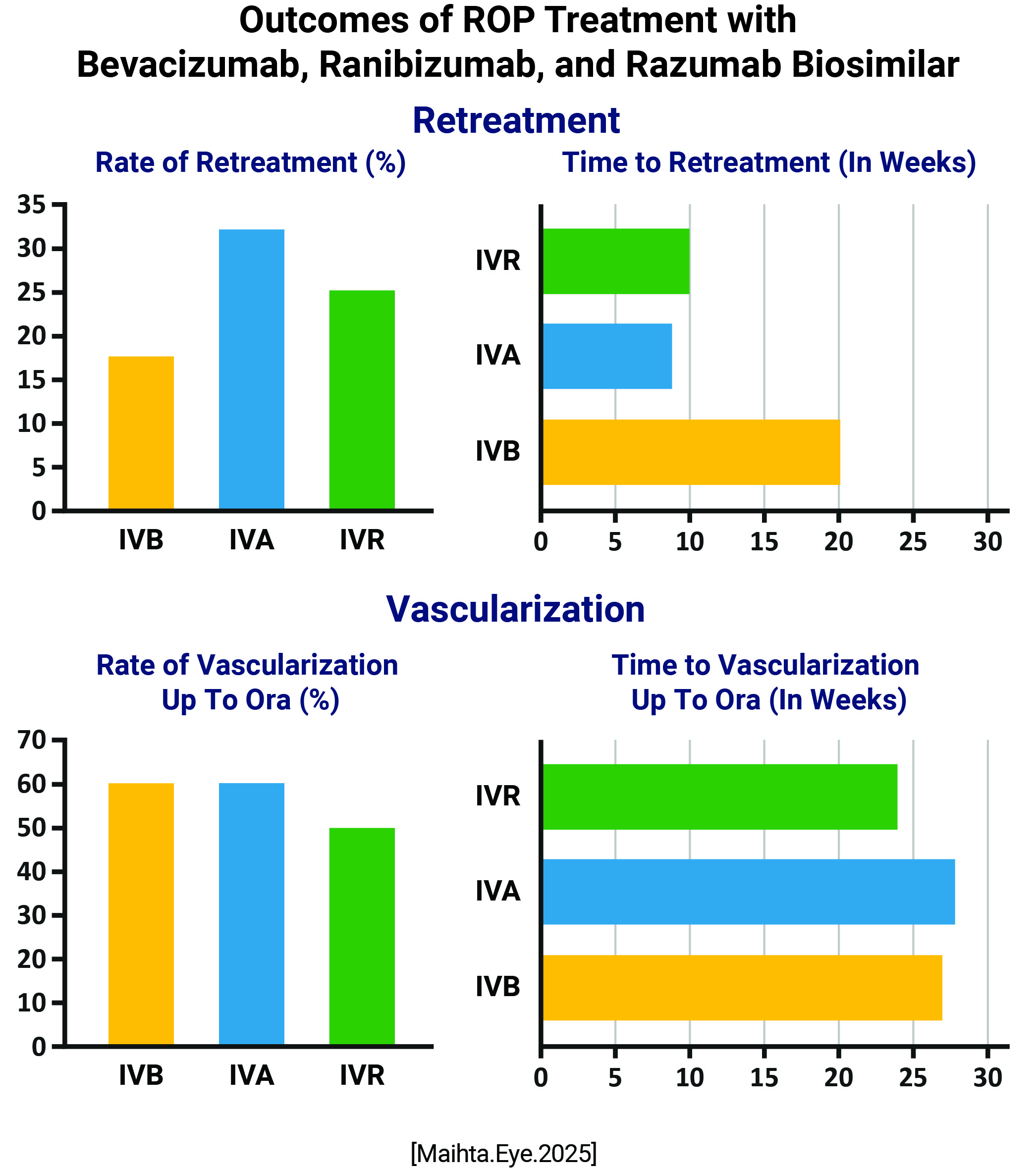 Rate of retreatment: IVB: 17.6% IVA: 32.2% IVR: 25% Time to retreatment: IVB: 20 weeks IVA: 8.8 weeks IVR: 10 weeks Vascularization Rates IVB: 60% IVA: 61% IVR: 50% Time to Vascularization IVB: 27 weeks IVA: 28 weeks IVR: 24 weeks No eyes developed intraocular inflammation or cataract. |
All three drugs showed comparable rates of retreatment, similar rates and timings of vascularization and good safety profile Bevacizumab showed significantly delayed reactivation due to pharmacokinetic differences; ranibizumab and its biosimilar razumab were comparable |
This study establishes the efficacy and safety profile beyond the regression of diseases, adding to our knowledge of reactivation and vascularization with the use of these molecules |
|
CRYO-ROP Multicenter trial of cryotherapy for retinopathy of prematurity: preliminary results. Cryotherapy for Retinopathy of Prematurity Cooperative Group |
Multicenter randomized trial |
1988: One eye of each patient with symmetrical ROP was randomly selected for transscleral cryotherapy.
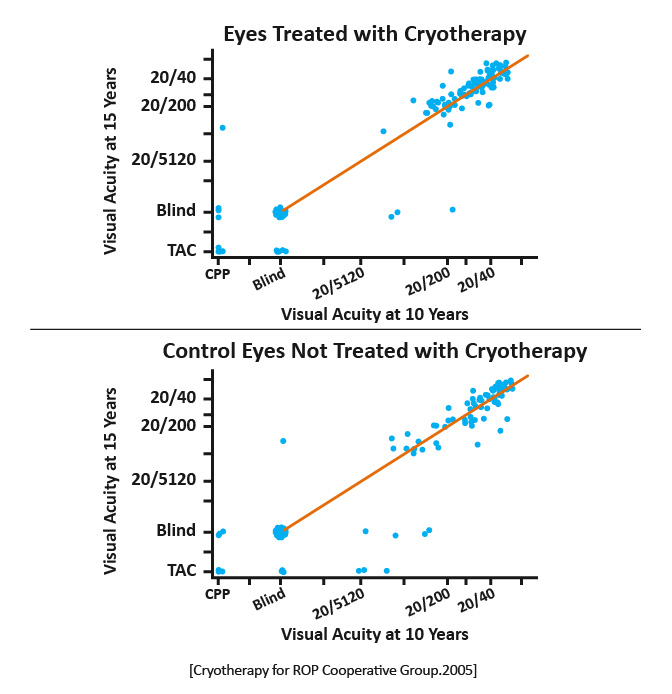
2005: Report of the ocular structure and visual acuity outcomes at age 15 years, and the incidence of retinal detachment between 10 and 15 years of age, for patients in the CRYO-ROP trial.
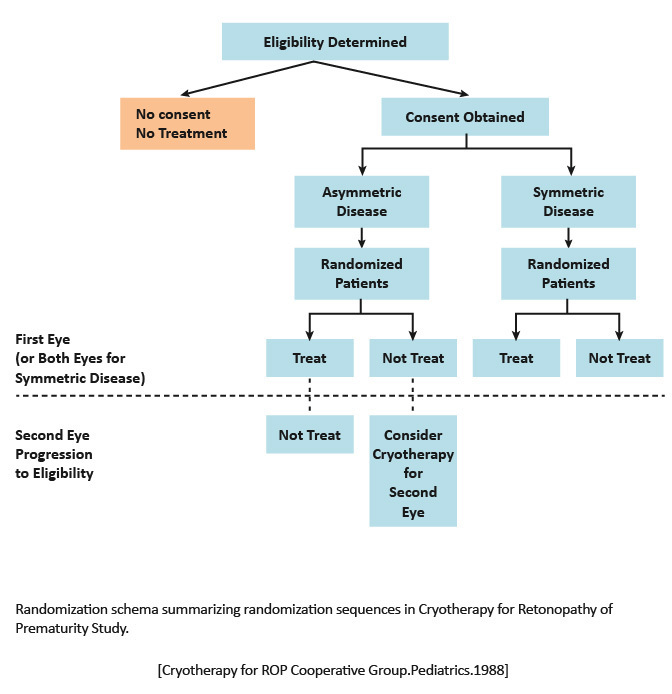
|
Structural and functional outcomes of transscleral cryotherapy for the treatment of ROP. |
1988: An unfavorable outcome was significantly less frequent in the eyes undergoing cryotherapy (21.8%) compared with the untreated eyes (43%).
2005: Thirty percent of treated eyes and 51.9% of control eyes had unfavorable structural outcomes.
|
1988: These data support the efficacy of cryotherapy in reducing by approximately one half the risk of unfavorable retinal outcome from threshold ROP. [Cryotherapy for ROP Cooperative Group.Pediatrics.1988] 2005: The benefit of cryotherapy for treatment of threshold ROP, for both structure and visual function, was maintained across 15 years of follow-up. New retinal detachments, even in eyes with relatively good structural findings at age 10 years, suggest value in long-term, regular follow-up of eyes that experience threshold ROP. [Cryotherapy for ROP Cooperative Group.ArchOphthalmol.2005] | 1988: Cryotherapy is recommended for at least one eye in symmetric ROP cases. For the fellow eye and for the single eye in asymmetric cases, the surgeon should use clinical judgment. [Cryotherapy for ROP Cooperative Group.Pediatrics.1988] 2005: Results from CRYO-ROP examinations conducted at 3.5, 5.5, and 10 years indicated that eyes that were saved from blindness by cryotherapy developed visual acuity that was better than 20/200 but worse than the normal range for age. [Cryotherapy for ROP Cooperative Group.ArchOphthalmol.2005] |
|
ETROP Final Results of the Early Treatment of Retinopathy of Prematurity (ETROP) Trial |
Randomized trial | Early retinal ablative treatment (n = 317) and the fellow eye managed conventionally (control eye) in infants with bilateral high-risk prethreshold ROP. For asymmetric cases (n = 84), the eye with high-risk prethreshold ROP was randomized to early retinal ablative treatment or to conventional management. | Visual acuity as assessed by masked testers using the Teller acuity card procedure. Structural examinations were performed at 6 and 9 months corrected age. | A reduction in unfavorable visual acuity outcomes occurred with earlier treatment, from 19.8% to 14.3%. Unfavorable structural outcomes were reduced from 15.6% to 9.0% at 9 months. | Early treatment of high-risk prethreshold ROP significantly reduced unfavorable outcomes in both primary and secondary/structural measures. [Good WV.Trans Am Ophthalmol Soc.2004] | It is possible to identify characteristics of ROP that predict which eyes are most likely to benefit from early peripheral retinal ablation. The clinical algorithm developed with this study provided treatment guidance for peripheral retinal ablation for infants with Type 1 and Type 2 ROP. [Good WV.Trans Am Ophthalmol Soc.2004] |
| Exploring An Interdisciplinary Support Model for ROP Exams in the NICU | Informal data collection and change in clinical practice | n/a |
Focuses on improving ROP screenings within Neonatal Intensive Care Units (NICUs). Proposes an interdisciplinary approach involving neonatologists, nurses, and ophthalmologists to enhance early ROP diagnosis and support. Aims to reduce stress and discomfort in preterm infants during ROP examinations |
After multiple iterations and testing, the final version was established as the new standard of care. The implementation process included staff education, protocol development, and ongoing evaluation to ensure adherence and effectiveness The new model resulted in increased family involvement and satisfaction, enhanced staff comfort and confidence during the exam process, and reduced negative clinical outcomes |
The importance of recognizing and responding to infants' behavioral cues, which is central to providing individualized, developmentally appropriate care The impact of sensitive caregiving on clinical outcomes, demonstrate that non-pharmacological pain and stress management interventions can significantly reduce stress and improve recovery in preterm infants The effective incorporation of families into the support process for stressful and painful procedures, highlights the role of parental involvement in promoting infant wellbeing The benefits of interdisciplinary collaboration in navigating complex healthcare systems to minimize discomfort and agitation during procedures through non-pharmacological interventions |
This model serves as a promising framework that can be adapted and implemented in other NICUs to enhance the quality of care for vulnerable infants |
| FIREFLEYE [Systemic exposure to aflibercept after intravitreal injection in premature neonates with retinopathy of prematurity: results from the FIREFLEYE randomized phase 3 study] | Randomized, open-label, noninferiority, phase 3 study | 0.4 mg intravitral injection of aflibercept 2 mg (n = 75 infants, 120/146 eyes) or laser photocoagulation (n = 38) | Endpoints include concentrations of free and adjusted bound aflibercept in plasma, pharmacokinetic/ pharmacodynamic exploration of systemic anti-VEGF effects, and immunogenicity |
Concentrations of free aflibercept were highly variable, with maximum concentration at day 1, declining thereafter Plasma concentrations of adjusted bound (pharmacologically inactive) aflibercept increased from day 1 to week 4, decreasing up to week 24 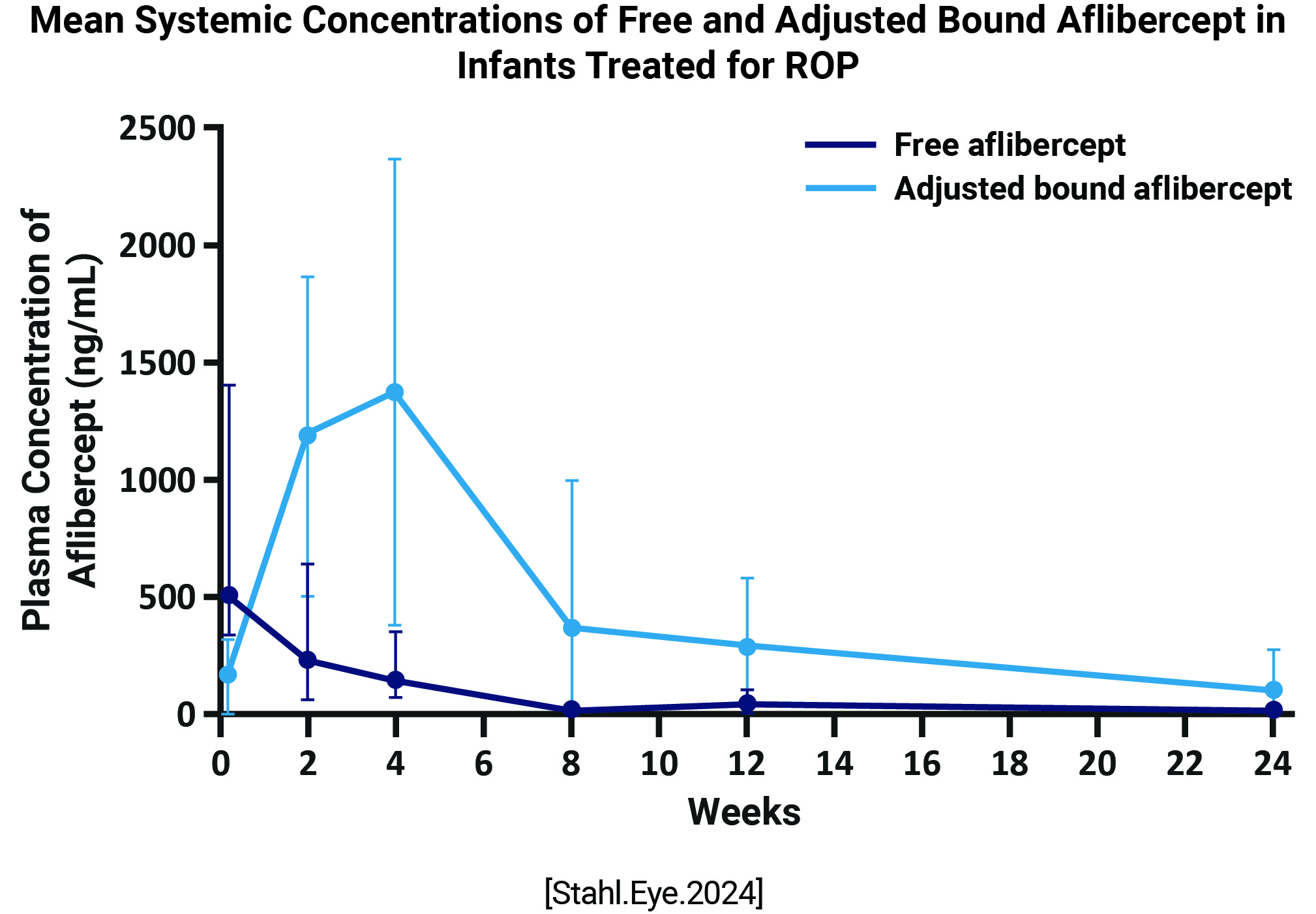 Six infants experienced treatment-emergent serious adverse events within 30 days of treatment; aflibercept concentrations were within the range observed in other infants. There was no pattern between free and adjusted bound aflibercept concentrations and blood pressure changes up to week 4 A low-titer (1:30), non-neutralizing, treatment-emergent anti-drug antibody response was reported in 1 infant, though was not clinically relevant |
Following intravitreal aflibercept (0.4 mg per eye) for treatment of ROP, concentrations of free and bound aflibercept 2 mg are not causally associated with clinically relevant effects of blood pressure or adverse events up to week 24 The clinically apparent AE profile was consistent with the established profile of intravitreal aflibercept 2 mg in adults |
The data provide no evidence suggestive of a causal association between aflibercept treatment and development of arterial hypertension or proteinuria |
|
RAINBOW Ranibizumab Versus Laser for the Treatment of Very Low Birthweight Infants With Retinopathy of Prematurity (RAINBOW): An Open-Label Randomised Controlled Trial |
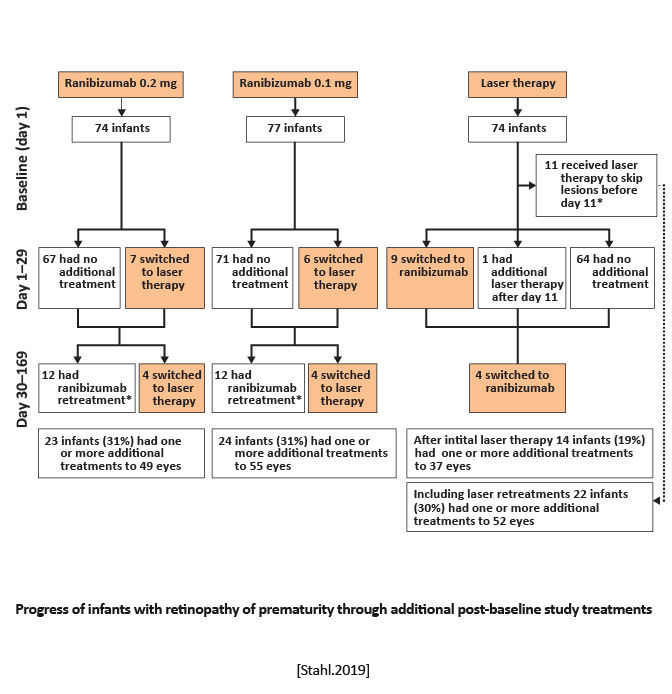
Randomized, open-label, superiority multicenter, three-arm, parallel group trial | Equal randomization (1:1:1) to receive a single bilateral intravitreal dose of ranibizumab 0.2 mg, ranibizumab 0.1 mg, or laser therapy. | Survival with no active retinopathy, no unfavorable structural outcomes, or need for a different treatment modality at or before 24 weeks. |
Treatment success occurred in 56 (80%) of 70 infants receiving ranibizumab 0.2 mg compared with 57 (75%) of 76 infants receiving ranibizumab 0.1 mg and 45 (66%) of 68 infants after laser therapy.
|
Ranibizumab 0.2 mg was as effective and safe in the treatment of active ROP as laser therapy, might be superior, and was associated with better short-term outcomes. [Stahl.Lancet.2019] | Intravitreal injection of ranibizumab can be considered as a novel treatment for ROP. However, disease recurrence must be monitored closely because it is common with anti-VEGF agents and can occur later compared with laser therapy. [Stahl.Lancet.2019] |
|
RAINBOW Ranibizumab Versus Laser for the Treatment of Very Low Birthweight Infants With Retinopathy of Prematurity (RAINBOW): An Open-Label Randomised Controlled Trial |
Randomized, open-label, superiority multicenter, three-arm, parallel group trial | Equal randomization (1:1:1) to receive a single bilateral intravitreal dose of ranibizumab 0.2 mg, ranibizumab 0.1 mg, or laser therapy. | Survival with no active retinopathy, no unfavorable structural outcomes, or need for a different treatment modality at or before 24 weeks. |
Treatment success occurred in 56 (80%) of 70 infants receiving ranibizumab 0.2 mg compared with 57 (75%) of 76 infants receiving ranibizumab 0.1 mg and 45 (66%) of 68 infants after laser therapy.

|
Ranibizumab 0.2 mg was as effective and safe in the treatment of active ROP as laser therapy, might be superior, and was associated with better short-term outcomes. [Stahl.Lancet.2019] | Intravitreal injection of ranibizumab can be considered as a novel treatment for ROP. However, disease recurrence must be monitored closely because it is common with anti-VEGF agents and can occur later compared with laser therapy. [Stahl.Lancet.2019] |