| Clinical Study “Common” Name (formal title) | Study design | Treatment arms | Primary outcomes | Results | Author conclusions | Real-world impact |
|---|---|---|---|---|---|---|
|
BALATON [A Phase III, Multicenter, Randomized, Double-Masked, Active Comparator-Controlled Study to Evaluate the Efficacy and Safety of Faricimab in Patients With Macular Edema Secondary to Branch Retinal Vein Occlusion] COMINO [A Phase III, Multicenter, Randomized, Double-Masked, Active Comparator-Controlled Study to Evaluate the Efficacy and Safety of Faricimab in Patients With Macular Edema Secondary to Central Retinal or Hemiretinal Vein Occlusion] |
Phase III, multicenter, randomized, double-masked, active comparator-controlled, parallel-group study in treatment-naïve patients with macular edema due to RVO |
BALATON: Aflibercept 2.0 mg (n=277) Faricimab 6.0 mg (n=276) COMINO: Aflibercept 2.0 mg (n=363) Faricimab 6.0 mg (n=366) |
Change from baseline in BCVA at week 24 |
At week 24 results were comparable between the two arms in each study.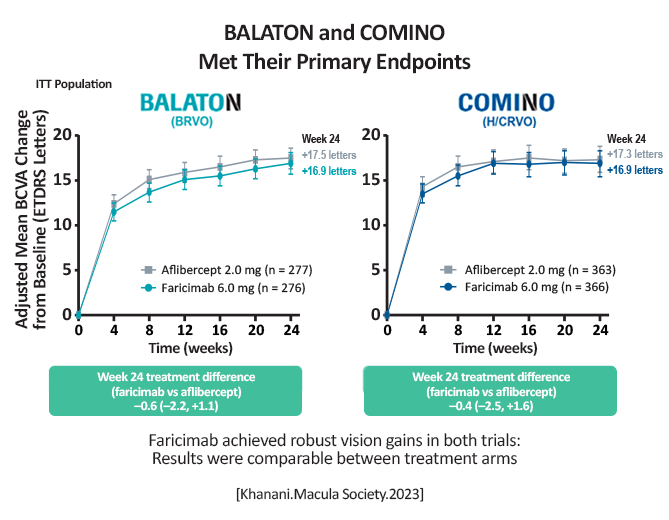
[Khanani.Macula Society.2023] BALATON: Faricimab: +16.9 letters; -311.4µm in central subfield thickness (CST) Aflibercept: +17.5 letters; -304.4µm in CST COMINO: Faricimab: +16.9 letters; -461.6µm in CST Aflibercept: +17.3 letters; -448.8µm in CST More patients achieved an absence of macular leakage with faricimab vs. aflibercept: BALATON, 33.6% vs. 21%, respectively (P = 0.0023) COMINO, 44.4% vs. 30%, respectively (P = 0.0002) [Khanani.Macula Society.2023] |
Faricimab met its primary endpoint and demonstrated a trend for advantages in absence of macular leakage. Faricimab was well tolerated, with a similar safety profile to aflibercept. [Khanani.Macula Society.2023] |
Faricimab is the first molecule to target 2 signaling pathways via the inhibition of angiopoietin-2 (Ang-2) and VEGF-A. |
|
BEACON [A Prospective, Randomized, Double-masked, Active Comparator-controlled, Multi-center, Two-arm, Phase 3 Study to Evaluate the Efficacy and Safety of Intravitreal KSI-301 Compared With Intravitreal Aflibercept in Participants With Visual Impairment Due to Treatment-naïve Macular Edema Secondary to Retinal Vein Occlusion (RVO)] |
Phase 3, prospective, randomized, double-masked, two-arm, multi-center non-inferiority study evaluating the efficacy and safety of repeated intravitreal dosing of tarcocimab 5 mg compared to aflibercept 2 mg in participants with visual impairment due to treatment-naïve macular edema secondary to RVO (either branch or central type). |
Tarcocimab dosed every 8 weeks Aflibercept dosed every 4 weeks |
Primary outcome was non-inferiority in change in BCVA from baseline to week 24 Tarcocimab: BRVO, n=220 CRVO, n=64 Aflibercept: BRVO, n=218 CRVO, n=66 |
At week 24, mean BCVA improved by 10.3 ETDRS letters in the tarcocimab group and by 11.2 ETDRS letters in the aflibercept group in BRVO.  [Shah.Retina Society.2023] Similar outcomes were reported for “All RVO”  [Shah.Retina Society.2023] Outcomes remained similar between the two groups from week 24 to week 48 Common ocular AEs were low and comparable between groups |
Tarcocimab treated patients had a ~30% higher chance of not requiring any additional doses versus aflibercept, even after receiving 2 fewer initiating doses (4 vs 6, respectively). Treatment burden distribution through 48 weeks had minimal overlap favoring tarcocimab, with 80% of tarcocimab patients receiving 5 or fewer doses vs 93% of aflibercept patients receiving 6 or more doses over 1 year After only 4 initiating doses in the first 6 months, approximately half of tarcocimab-treated patients required no additional injections through 12 months of treatment. [Shah.Retina Society.2023] |
Tarcocimab is an anti-VEGF antibody biopolymer conjugate (ABC) that blocks all VEGF-A isoforms. It may provide a viable option to currently marketed anti-VEGF treatments as BEACON showed it has similar outcomes to aflibercept, but with a much longer duration of treatment. |
| BRAVO, (RanibizumaB for the Treatment of Macular Edema following BRAnch Retinal Vein Occlusion) | Prospective, randomized, sham injection-controlled, double-masked, multicenter trial | Ranibizumab 0.3mg (n-134) Ranibizumab 0.5 mg (n=131) Sham (n=132) | Mean change from baseline BCVA letter score at month 6 | The mean change from baseline BCVA letter score was 16.6 in the 0.3 mg ranibizumab group, 18.3 in the 0.5 mg ranibizumab group, and 7.3 in the sham group (P<0.0001 for each ranibizumab group vs sham). | Monthly intravitreal ranibizumab injections provided important benefits for patients with macular edema following RVO, but several important questions remained, notably what percentage of patients would remain edema-free after ranibizumab treatment discontinuation as well as the impact of delays or gaps in treatment. [Campochiaro.2010] As-needed injections of ranibizumab maintained the improvement in visual acuity and central foveal thickness seen at month 6. | This was the first study to demonstrate anti-VEGF as an alternative to laser treatment for BRVOs with macular edema. The study design did not randomize patients to laser on day 0 so there is no direct head-to-head comparison. Nevertheless, treatment with laser resulted in suboptimal visual outcomes even when the patients were transitioned to anti-VEGF implying the 6 month delay in treatment is a significant concern. [Brown.2011] |
| BRVO (Comparing the Efficacy of Bevacizumab and Ranibizumab in Patients with Retinal Vein Occlusion) | Comparative, randomized, double-masked, multicenter, noninferiority clinical trial. | Bevacizumab 1.25 mg (n=144) Ranibizumab 0.5 mg (n-142) 133 diagnosed with BRVO 97 diagnosed with CRVO 47 diagnosed with hemi-CRVO | Change in BCVA of the study eye from baseline to 6 months, with a noninferiority margin of 4 letters. |
At 6 months, overall: BRVO Bevacizumab : 14.2 letter gain Bevacizumab : 16.1 letter gain |
Monthly administration of bevacizumab 1.25 mg is noninferior to monthly administration of ranibizumab 0.5 mg through month 6 in the treatment of patients with macular edema resulting from RVO. [Vader.2020] |
Patients in this study were treated monthly for 6 months, which is more than most real-world clinics will do. However, this adds to the literature and re-confirms noninferiority between bevacizumab and ranibizumab. |
| BVOS (Branch Vein Occlusion Study) | Multicenter, randomized trial | Group 1 included eyes at risk for the development of neovascularization, [BVOS.1984] group 2 included eyes at risk for the development of vitreous hemorrhage, [BVOS.1984] and group 3 included eyes at risk for vision loss from macular edema. [BVOS.1984] Group 3: Grid laser (n=71) No laser (n=68) |
For group 3, efficacy of macular grid laser for the treatment of macular edema secondary to BRVO
(Of note, there were no specific primary endpoints) |
At 3 years, the efficacy of laser treatment was clear; treated eyes in group 3 gained an average of 1.33 lines compared with 0.23 lines in a control group. [BVOS.1984] | Argon laser photocoagulation improved VA in patients with 20/40 or worse. Study authors recommended the treatment for those with VA reduced as a result of macular edema, not for those whose vision loss was a result of intraretinal hemorrhage in the fovea or fovea capillary nonperfusion. [BVOS.AJO.1984/p281/col1/para3] | Based on these data, macular grid laser photocoagulation became the first-line treatment for patients with macular edema following RVO. Laser treatment is considered a second-line treatment today only if patients are contraindicated for an anti-VEGF or if they are unlikely to keep up with monthly treatments. [Flaxel.2020] |
|
VIBRANT [Study to Assess the Clinical Efficacy and Safety of Intravitreal Aflibercept Injection (IAI;EYLEA;BAY86-5321) in Patients With Branch Retinal Vein Occlusion (BRVO) |
Phase 3, multicenter, randomized, double-masked, active-controlled trial | Macular grid laser photocoagulation (n=92) Aflibercept 2mg Q4 weeks (n=91) |
Treatment to week 20 Primary endpoint: proportion of patients 15 letters in BCVA from baseline [Campochiaro.2015] |
Week 24: Macular grid laser photocoagulation: 26.7% Aflibercept 2mg Q4 weeks: 52.7% [Campochiaro.2015] Week 52: Macular grid laser photocoagulation: 41.1% Aflibercept 2mg Q4 weeks: 57.1% [Clark.2016] | Treatment with aflibercept 2 mg every 4 weeks was superior to laser treatment through week 24 in visual acuity gains and anatomic outcomes. [Clark.2016] Continued treatment with an every 8-week regimen maintained visual and anatomic gains through week 52 after 6 monthly injections. [Clark.2016] | The study randomized patients in a head-to-head fashion on day 0 to either laser or aflibercept treatment.The results further validated that anti-VEGF treatment is superior to laser treatment. [Clark.2016] |