| Clinical Study “Common” Name (formal title) | Study design | Treatment arms | Primary outcomes | Results | Author conclusions | Real-world impact |
|---|---|---|---|---|---|---|
| COPERNICUS (Controlled Phase 3 Evaluation of Repeated Intravitreal Administration of VEGF Trap-Eye in Central Retinal Vein Occlusion: Utility and Safety) | Prospective, randomized trial | Aflibercept 2mg (n=115) Sham (n=74) |
The proportion of eyes with a 15-letter gain or more in best-corrected visual acuity (BCVA) at week 24;
Beginning at week 24, patients in both groups were eligible to receive aflibercept 2 mg PRN through week 100 [Brown. 2013] [Heier.2014] |
Week 24: Aflibercept 2mg: 66/114; 57.9%
Sham: 9/74;12.3% Aflibercept- treated eyes gained a mean of 17.3 letters versus sham-treated eyes, which lost 4.0 letters Week 52: Aflibercept 2mg: 55.3% Sham + Aflibercept 2 mg: 30.1% Week 100: Aflibercept 2mg: 49.1% Sham + aflibercept 2 mg: 23.3% [Heier.2014] [Boyer.2012] |
Monthly intravitreal injection of aflibercept 2 mg in eyes with macular edema resulting from CRVO improved visual acuity and central retinal thickness, eliminated progression resulting from neovascularization, and was associated with a low rate of ocular adverse events related to treatment. [Boyer.2012] | The study validated that a 24-week delay in treatment resulted in a significant reduction in vision gains despite continued anti-VEGF treatment. In addition, the retinal perfusion status increased in those treated with anti-VEGF over time suggesting that some of the retinal vein occlusion perfusion deficits are reversible. [Heier.2014] |
| CRAVE (Comparison of Anti-VEGF Agents in the Treatment of Macular Edema from Retinal Vein Occlusion Trial) | Prospective randomized trial | Bevacizumab 1.25 mg (n=19) Ranibizumab 0.5 mg (n=20) | Change in central foveal thickness (CFT) at month 6 | Mean reduction of 212.6 ¼m in the bevacizumab group Mean reduction of 243.8 ¼m in the ranibizumab group No significant difference in the number of patients who achieved CFT >275 ¼m [Rajagopal.2015] | No statistically significant differences between bevacizumab and ranibizumab in treatment of RVO in either anatomic or visual outcomes. [Rajagopal.2015] | CRAVE set the stage for the comparison between bevacizumab and aflibercept (SCORE 2) |
| CRUISE (Ranibizumab for the Treatment of Macular Edema after Central Retinal Vein OcclUsIon Study: Evaluation of Efficacy and Safety) | 6-month, phase 3, multicenter, randomized, injection-controlled study in 2 phases: day 0 to month 6, and months 6-12 | Sham injection (n=130) Ranibizumab 0.3 mg (n=132) Ranibizumab 0.5 mg (n=130) | Change from baseline BCVA letter score at month 6 |
6-month results:
Sham injection: 0.8 letters Ranibizumab 0.3 mg: 12.7 letters Ranibizumab 0.5 mg: 14.9 letters 12-month results: Sham injection: 7.3 letters (of note, patients could be treated with ranibizumab in the second 6 months) Ranibizumab 0.3 mg: 13.9 letters Ranibizumab 0.5 mg: 13.9 letters |
The percentage of patients who gained 15 letters increased rapidly after injection of ranibizumab (26.9% in the 0.5 mg group) compared with 3.8% in the sham group at day 7 (P<0.0001). [Brown.2010] PRN treatment months 6-11 maintained visual and anatomic outcomes. [Campochiaro.2011] | First study to demonstrate that anti-VEGF lead to meaningful visual outcomes for patients with a CRVO and macular edema. Patient transitioned from sham to 0.5 mg of Ranibizumab at month 6 had less visual and anatomic gains than those treated initially. Only ranibizumab 0.5 mg is approved for the treatment of CRVO. [Lucentis.PI.2018] |
| CVOS (Central Vein Occlusion Study) | Multicenter, randomized trial | Macular grid laser photocoagulation (n=77) No laser treatment (n=78) | Efficacy of laser treatment for patients with macular edema following CRVO with VA of 20/50 or worse. | There was no visual benefit of laser treatment in patients with CRVO and macular edema. [CVOSG.1995] | CVOS researchers could not recommend laser treatment in this patient population. [CVOSG.1995] | Because laser treatment did not improve VA in this patient population, observation remained the standard of care in the management of CRVO. The study did not evaluate intravitreal steroids for treatment of macular edema from CRVO. |
| GALILEO (General Assessment Limiting Infiltration of Exudates in Central Retinal Vein Occlusion with VEGF Trap-Eye) | Prospective, randomized trial | Aflibercept 2mg (n=106) Sham (n=71) *** GALILEO differed from COPERNICUS in that patients initially randomized to the sham control group remained on sham control from weeks 24 to 52. [Holz.2013] | Proportion of patients with 15 letter gain from baseline to week 24 | Week 24: Aflibercept 2 mg: 60.2% Sham: 22.1% Week 52: Aflibercept 2 mg: 60.2% Sham: 32.4% Week 100: Aflibercept 2mg: 57.3% Sham + aflibercept 2 mg: 29.4% [Korobelnik.2014] [Holz.2013] [Ogura.2014] | Aflibercept 2 mg every 4 weeks led to significantly better visual acuity outcomes than sham. Improvement was seen as early as the first posttreatment assessment at week 4 and stabilized around week 16. [Holz.2013] These results were consistent with those seen in COPERNICUS. [Holz.2013]The PRN phase of GALILEO determined that the improvements in BCVA and central retinal thickness achieved with monthly aflibercept injections in the first 24 weeks were largely maintained with monthly monitoring. [Korobelnik. 2014] | Treatment intervals can be extended after treatment initiation with monthly doses but monitoring and treatment intervals must be individualized to the patient based on visual and anatomic outcomes. [Ogura.2014] |
| LEAVO (Lucentis, Eylea, Avastin in Vein Occlusion Study) | Prospective, 3-arm, double-masked, randomized noninferiority trial | Aflibercept 2 mg (n=154) Bevacizumab 1.25 mg (n=154) Ranibizumab 0.5 mg (n=155) | Adjusted mean change in BCVA in the study eye at 100 weeks | At 100 weeks: Aflibercept 2 mg: 15.1 letter gain Bevacizumab 1.25 mg: 9.8 letter gain Ranibizumab 0.5 mg: 12.5 letter gain [Hykin.2019] | Aflibercept was noninferior to ranibizumab, but bevacizumab was not noninferior (different) to ranibizumab and aflibercept [Hykin.2019] | Statistically, aflibercept and ranibizumab were considered equal although the vision outcomes were greater with aflibercept than ranibizumab and bevacizumab. More patients with aflibercept had a dryer retina after treatment (CST<320) than ranibizumab or bevacizumab. [Hykin.2019] |
| SCORE2 (Study of COmparative Treatments for REtinal Vein Occlusion 2) | Multicenter, prospective, randomized, phase 3 trial | Bevacizumab 1.25 mg (n=180) Aflibercept 2.0mg (n=182) Both arms dosed every 4 weeks | Change from baseline in VA letter score (VALS), VALS gain of 5 letters change from baseline in central subfield thickness (CST), CST >300 ¼m, and resolution of macular edema | VALS gain of 15 letters: Bevacizumab: 106/182 eyes (61.3%) Aflibercept: 114/180 eyes (65.1%) [Scott. 2017] | Aflibercept treatment was associated with more favorable spectral domain optical coherence tomography outcomes but not VA outcomes. [Scott.2017] | Aflibercept and Bevacizumab are non-inferior for the treatment of macular edema secondary to retinal vein occlusion with a small number of patients requiring present protocol criteria for rescue with dexamethasone at month 6. Why the difference with the LEAVO study? The study visions were worse in SCORE, OCTs were thicker, and the patients had their RVOs for longer.These factors may have contributed to the difference in outcomes between these two studies. [Scott. 2017] |
|
SCORE2 Year 5 (Month 60 Outcomes After Treatment Initiation With Anti–Vascular Endothelial Growth Factor Therapy for Macular Edema Due to Central Retinal or Hemiretinal Vein Occlusion) |
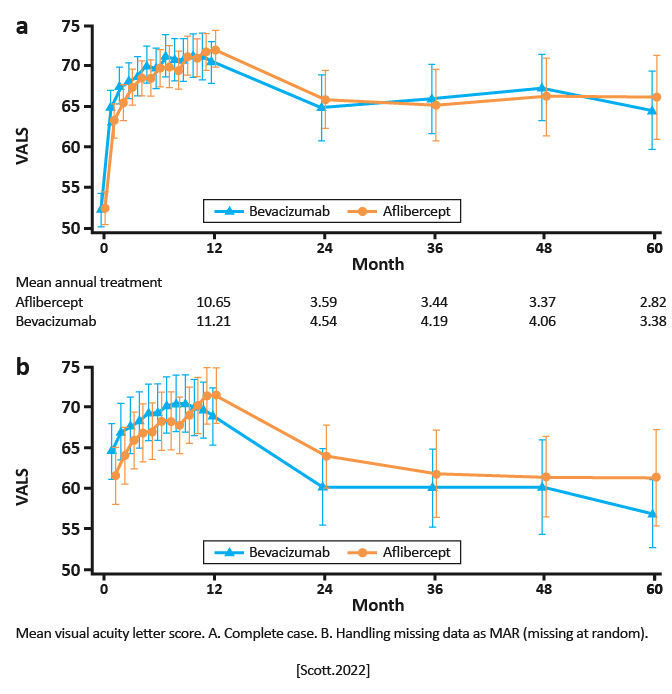
Long-term follow-up (up to 60 months) after a randomized clinical trial from 64 centers in the US. | After primary outcome assessment at month 6, participants originally assigned to aflibercept who met the protocol-defined criteria for a good response were rerandomized to either continuing aflibercept every 4 weeks (n=79) vs changing to a treat and extend regimen (n=80); 15 participants with a protocol-defined poor or marginal response at 6 months were assigned to receive a dexamethasone implant. Participants originally assigned to bevacizumab who met the protocol-defined criteria for a good response were rerandomized to either continuing bevacizumab every 4 weeks (n=67) vs changing to a treat and extend regimen (n=67); participants (n=39) with a protocol-defined poor or marginal response at 6 months were assigned to receive aflibercept. | Visual acuity letter score (VALS) and central subfield thickness on optical coherence tomography after 60 months of follow-up in eyes initially treated with aflibercept or bevacizumab for macular edema due to central retinal or hemiretinal vein occlusion. |
Among participants completing month 60, overall mean VALS improvement over baseline was 13.5 (95% CI: 9.6, 17.5), less than the mean improvement of 20.6 (95% CI: 18.7, 22.4) observed at month 12, with no significant differences between originally assigned study groups.
|
Although VALS improved substantially when patients were treated per protocol through month 12, improvement lessened when treatment was at investigator discretion and fewer treatments were received although VALS remained markedly improved over baseline through year 5. [Scott.AmJOphthalmol.2022] | As most patients in this study continued to receive treatment in year 5, continued monitoring and, if warranted, treatment with anti-VEGF therapy benefits patients with macular edema associated with central retinal or hemiretinal vein occlusion. [Scott.AmJOphthalmol.2022] |