| Clinical Study “Common” Name (formal title) | Study design | Treatment arms | Primary outcomes | Results | Author Conclusions | "Real-world" impact |
|---|---|---|---|---|---|---|
|
ATLANTIC (Efficacy and Safety of Intravitreal Aflibercept Treat and Extend for Polypoidal Choroidal Vasculopathy in the ATLANTIC Study: A Randomized Clinical Trial) |
Double-masked, sham-controlled, investigator-initiated randomized trial | Patients were randomized at week 16 to receive intravitreal aflibercept injection (IVAIs) in a treat and extend (T&E) regimen plus either sham photodynamic therapy (PDT) or standard fluence PDT with verteporfin. | Changes in best corrected visual acuity (BCVA) from baseline to 52 weeks and polyp occlusion at week 52 in nave symptomatic Caucasian patients with polypoidal choroidal vasculopathy. |
A significant median BCVA gain of 6 [212] Early Treatment Diabetic Retinopathy Study letters was observed for all patients P< .001), after 8 (79) injections, with a significant reduction of 93.0 [154.0, 44.0]m in central macular thickness (P< .001). Using indocyanine green angiography, a complete occlusion of polypoidal lesions was documented in 72% of the cases. Still, no significant difference was detected between the sham PDT and the aflibercept PDT arms, at week 52 for BCVA change, number of IVAIs, change in central retinal thickness, and rates of complete polyp occlusion.
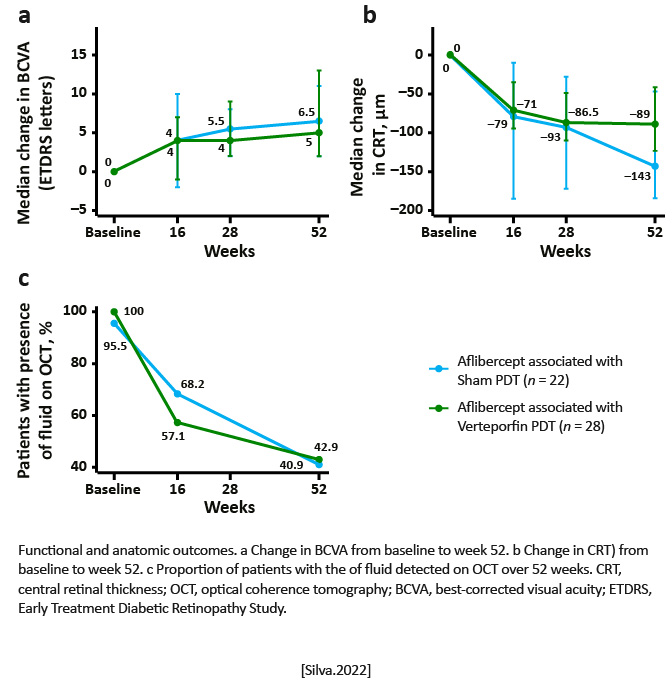
|
To the authors™ knowledge, this is the first RCT to compare aflibercept T&E monotherapy with aflibercept T&E plus verteporfin PDT in a Caucasian population with PCV. Aflibercept monotherapy in T&E showed to be effective and safe with a significant median BCVA improvement of 6 letters and a complete occlusion of polypoidal lesions in near three quarters of the eyes, at 1 year. [Silva. Ophthalmologica.2022] | As only 22% of the eyes underwent PDT treatment, the benefit of combined treatment for PCV in Caucasians could not be definitively elucidated from this study. [Silva. Ophthalmologica.2022] |
| EVEREST (Efficacy and Safety of Verteporfin Added to Ranibizumab in the Treatment of Symptomatic Macular Polypoidal Choroidal Vasculopathy) | Multicenter, randomized active controlled, double-masked, indocyanine green angiography (ICGA) guided exploratory study in Asian patients |
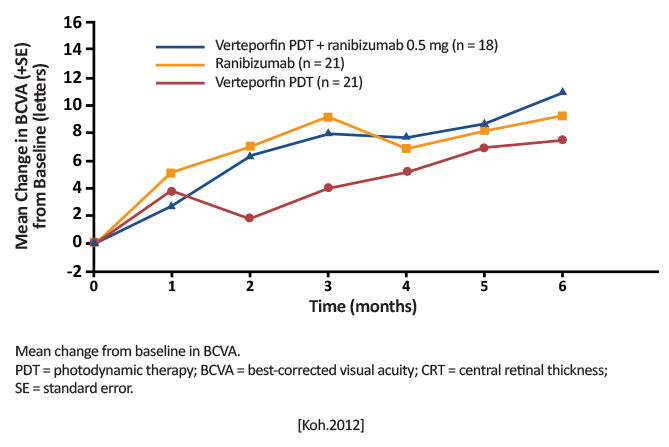
Arm 1: Verteporfin PDT (6 mg/m2) + ranibizumab 0.5 mg (n=18) Arm 2: Verteporfin PDT (6 mg/m2) + sham injection (n=21) Arm 3: ranibizumab 0.5 mg + sham PDT (n=21) [Koh.Retina.2012] |
Proportion of patients achieving complete regression of polyps at Month 6 [Koh.Retina.2012] |
Month 6: Arms 1 and 2 were superior to Arm 3 in achieving complete polyp regression (77.8% and 71.4% vs 28.6%; P< .01)
[Koh.Retina.2012]
|
Verteporfin PDT with or without ranibizumab is superior to ranibizumab monotherapy in achieving complete regression of polyp in patients with PCV. First trial to use ICGA-assessed polyp closure as basis for retreatment. [Koh.Retina.2012] |
First time the ICGA criteria were used for PCV diagnosis, suggesting imaging can be a useful clinical guideline [Koh.Retina.2012] |
| EVEREST II (Efficacy and Safety of Ranibizumab With or Without Verteporfin Photodynamic Therapy for Polypoidal Choroidal Vasculopathy: A Randomized Clinical Trial) |
24-month, multicenter, randomized, double-masked study [Koh.JAMA Opthalmology.2017] |
Combination therapy (ranibizumab 0.5 mg and verteporfin PDT [n=168]) Monotherapy (ranibizumab 0.5 mg and sham PDT [n=154]) [Koh.JAMA Ophthalmology.2017] |
Two parts:
|
At 12 months Combination therapy: +8.3 letters Monotherapy: +5.1 letters Complete polyp regression Combination therapy: 69.3% Monotherapy: 34.7% P< .001 [Koh.JAMA Ophthalmology.2017] |
Combination therapy is noninferior/ superior to ranibizumab monotherapy in BCVA and complete polyp regression [Koh.JAMA Ophthalmology. 2017] |
Combination therapy reduces treatment burden and is more effective than ranibizumab therapy alone both in terms of visual and anatomic outcomes. [Koh.JAMA Ophthalmology. 2017] |
| EVEREST II (Comparison of Ranibizumab With or Without Verteporfin Photodynamic Therapy for Polypoidal Choroidal Vasculopathy: The EVEREST II Randomized Clinical Trial) | Double-masked, multicenter, randomized clinical trial |
Combination group [Ranibizumab plus PDT (n=168)] Ranibizumab monotherapy (n=154) [Lim.JAMA.2020] |
Follow-up to Month 12; prespecified secondary endpoints:
|
Month 24 BCVA: Combination group: +9.6 letters Monotherapy group: +5.5 letters Complete polypoidal lesion regression Combination group: 81/143; 56.6% Monotherapy: 23/86; 26.7% P< .001) [Lim.JAMA.2020] 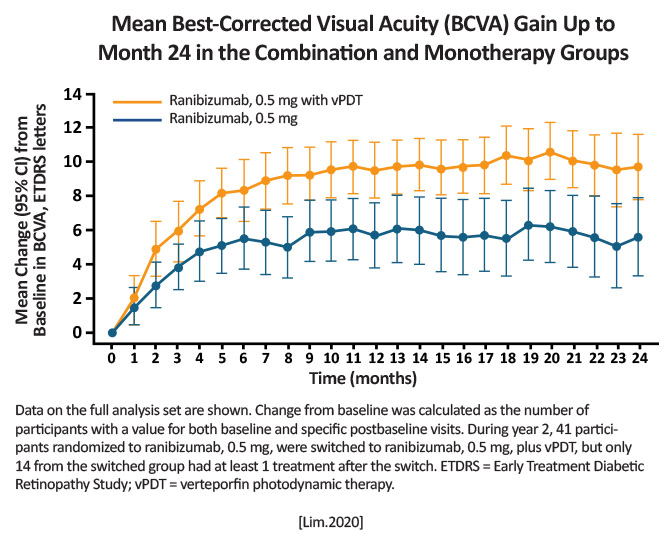
|
Combination treatment is superior in both BCVA gains and complete lesion resolution at 24 months compared to monotherapy. [Lim.JAMA.2020] |
Combination therapy through Month 24 maintains the gains reported at Month 12, while continuing to reduce patient treatment burden. [Lim.JAMA.2020] |
| LAPTOP Study: A 24-Month Trial of Verteporfin Versus Ranibizumab for PolypoidalChoroidal Vasculopathy |
Randomized controlled trial
|
PDT arm (n=47): single session of PDT with verteporfin Ranibizumab arm (n=46): 3 monthly ranibizumab injections at baseline |
Proportion of patients gaining or losing more than 0.2 logarithm of minimal angle of resolution (logMAR) units from baseline [Oishi.Am J Ophthalmol.2013] |
Baseline and final logMAR: Ranibizumab arm: 0.48±0.27 and 0.40 ±0.37 PDT arm: 0.57±0.31 and 0.65±0.46 P= .004 [Oishi. Ophthalmology. 2014] |
Ranibizumab produced better 24-month results than PDT. [Oishi. Ophthalmology. 2014] |
Adds data to support ranibizumab for the treatment of PCV, but the study was unable to include a combination arm. In this study, anatomic improvements were better with PDT than ranibizumab, but continual treatment with ranibizumab can prevent further vision loss. |
| PLANET (Aflibercept in Polypoidal Choroidal Vasculopathy) | Double-masked, sham-controlled randomized phase 3b/4 trial |
All arms: 2 mg of aflibercept at weeks 0, 4, and 8 (n=318) Week 12: |
Change in BCVA from baseline to Week 52 [Lee.JAMA Ophthalmology. 2018] |
Mean BCVA gain from baseline to Week 52: Aflibercept monotherapy: +10.7 letters Aflibercept and PDT: +11.3 letters [Lee.JAMA Ophthalmology.2018] |
Visual acuity (VA) and/or functional outcomes improved in 85% of patients treated with aflibercept monotherapy. No signs of leakage from lesions in 80%+ [Lee.JAMA Ophthalmology. 2018] |
Confirmed the efficacy of aflibercept in patients with PCV, but it could not confirm the benefit of adding PDT because too few patients received it. [Lee.JAMA Ophthalmology. 2018] |
| Three-Year Visual Outcomes of Intravitreal Ranibizumab With or Without Photodynamic Therapy for Polypoidal Choroidal Vasculopathy | Retrospective, single center, nonrandomized trial |
Ranibizumab monotherapy (n=20) Combination therapy with PDT and ranibizumab (n=25) [Sakai.Acta Ophthalmologica.2016] |
Mean change in BCVA, number of ranibizumab injections, and length of treatment-free period over 36 months [Sakai.Acta Ophthalmologica.2016] |
BCVA (logMAR) from baseline to 36 months: Monotherapy: 0.410.31 letters to 0.480.40 letters Combination: 0.470.22 letters to 0.260.30 letters 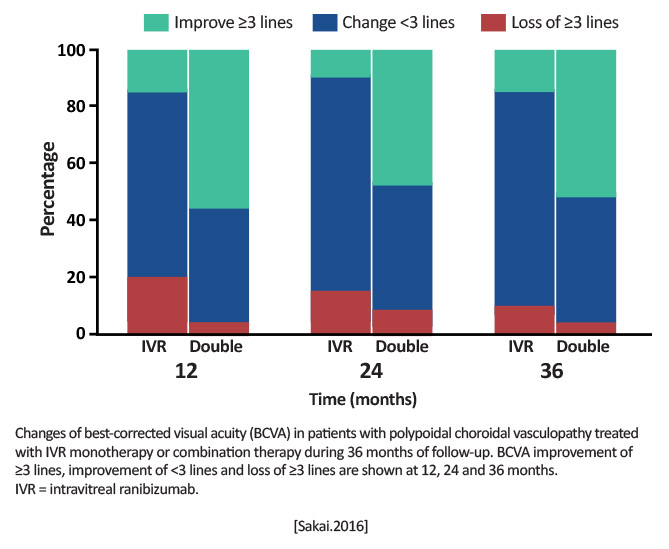
|
Combination therapy was more effective than monotherapy in patients with PCV. [Sakai.Acta Ophthalmologica.2016] |
Combination therapy may extend the treatment-free period and decrease the need for additional anti-VEGF injections. |
| Treatment of Polypoidal Choroidal Vasculopathy by Photodynamic Therapy, Aflibercept and Dexamethasone Triple Therapy |
Prospective, consecutive case series [Ho.Sci Rep.2016] |
All eyes (n=17) were treated with PDT, intravitreal aflibercept 2 mg, and intravitreal dexamethasone 600 ¼g/0.15 ml [Ho.Sci Rep.2016] |
BCVA at 6 months and 1 year [Ho.Sci Rep.2016] |
6 months: VA stable at 20/50 Central foveal thickness (CFT) stable at 259 m Lesion resolution in 13 eyes (82%) 1 year: Average VA at 20/40 CFT was 280 m 2 eyes/2 patients (12%) had a recurrence (6 and 8 months) 12 eyes (70.5%) had complete resolution of pigment epithelial detachment [Ho.Sci Rep.2016] |
Triple therapy with PDT, aflibercept, and dexamethasone is an effective treatment for PCV. [Ho.Sci Rep. 2016] |
First attempt at triple therapy seems to be beneficial, but the sample size was small, and some patients still had a recurrence. |
| VAULT (Effect of Intravitreal VEGF-Trap Eye on Polypoidal Choroidal Vasculopathy) | Prospective, single-arm, interventional case series | Intravitreal aflibercept in 3 monthly doses, followed by maintenance injection every 2 months for 1 year (total of 7 injections) [Lee.Graefes Arch Clin Exp Ophthalmology.2017] |
Proportion of patients who maintained BCVA (losing <15 ETDRS letters) [Lee.Graefes Arch Clin Exp Ophthalmology.2017] |
12 months: BCVA maintained or improved in 87.5% (35 of 40 eyes); average gain +9 letters; 14 eyes (33.3%) had fluid recurrence 26 eyes (66.7%) had complete polyp regression [Lee.Graefes Arch Clin Exp Ophthalmology.2017] 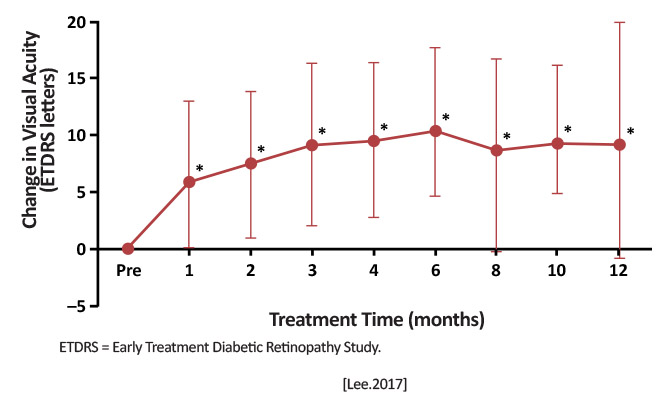
|
Fixed dosing aflibercept showed favorable outcomes at 12 months in both anatomical and functional end points. [Lee.Graefes Arch Clin Exp Ophthalmology.2017] |
Some patients with PCV will have good outcomes on a fixed-dose, bimonthly injection schedule with aflibercept after 3 loading doses, suggesting it may be another potential treatment. |